Facile fabrication of graphene composite microwires via drying-induced size reduction of hydrogel filaments†
Abstract
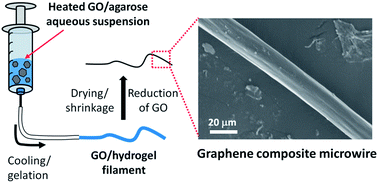
Graphene based electrical conductors are under consideration for numerous applications in energy storage, energy conversion devices, electronics and sensors. Here, we report a facile and versatile method for fabrication of graphene based composite microwires ranging from ∼20 to 250 μm in diameter via size reduction during dehydration of extruded larger diameter graphene oxide-loaded agarose hydrogel fibers. The graphene oxide is effectively reduced to graphene by chemical and thermal treatment. After dehydration and reduction, the resulting graphene composite microwires exhibit high electrical conductivities of up to 1.8 S m−1.

 Please wait while we load your content...
Please wait while we load your content...