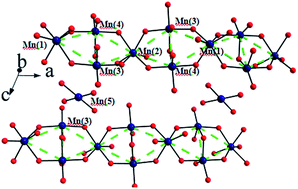
KNa3Mn7(PO4)6: 2D spin-frustrated magnetic material with a diamond-like chain structure†
Abstract
The search for spin frustrated magnets has attracted great scientific interest. Here we report on the synthesis of a novel two dimensional spin-frustrated compound KNa3Mn7(PO4)6 with a diamond-like chain structure. Our results confirm spin freezing at Tc = 3.9(1) K with the Weiss constant θ = −64.1(2) K, showing a remarkable spin frustration effect in the system. The origin of spin frustration is also suggested.

 Please wait while we load your content...
Please wait while we load your content...