Separation of BTEX aromatics from n-octane using a (tetrabutylammonium bromide + sulfolane) deep eutectic solvent – experiments and COSMO-RS prediction
Sarwono Mulyonoa,
Hanee F. Hizaddinb,
Inas M. Alnashefa,
Mohd A. Hashimb,
Anis H. Fakeehaa and
Mohamed K. Hadj-Kali*a
aDepartment of Chemical Engineering, College of Engineering, King Saud University, P. O. Box 800, Riyadh 11421, Saudi Arabia. E-mail: mhadjkali@ksu.edu.sa; Tel: +966114676040
bDepartment of Chemical Engineering, Faculty of Engineering, University of Malaya, 50603 Kuala Lumpur, Malaysia
First published on 20th February 2014
Abstract
Separation of aromatics from aliphatics is a challenging process because of the close range of their boiling points and the formation of several combinations of azeotropes. Until now, no feasible separation process is available for aromatic concentrations below 20 wt%. In this work, we have investigated the possibility of using a selected deep eutectic solvent (DES) for the liquid–liquid extraction of benzene, toluene, ethylbenzene and m-xylene (BTEX) aromatics. The DES used in this work was synthesized by combining tetrabutylammonium salt and sulfolane. Equilibrium data for the ternary system consisting of BTEX aromatics, n-octane and DES were measured at 25 °C and atmospheric pressure. The results showed that the used DESs have comparable distribution ratios and selectivities to those of commercial solvents. In all tested systems, sulfolane was not present in the hydrocarbon layer. It was also found that the selectivity decreases with decreasing polarity of the aromatic compound. The Non-Random Two Liquid (NRTL) model was successfully used to correlate the experimental tie-lines and to calculate the phase compositions of the ternary systems. In addition, the performance of COSMO-RS to predict the ternary tie-lines for the studied systems was evaluated and the σ-profiles were used to explain the interaction between the DES and the aromatic compounds.
Introduction
Benzene, toluene, ethylbenzene and xylene (BTEX) are important chemicals for industry; they are used as raw materials in producing plastic and polymers as well as intermediates in the synthesis of many other chemicals. The primary source of these aromatics is the catalytic reforming of naphtha. During the reforming process, low-octane number straight-run naphtha is converted into high-octane gasoline called reformate.1 The BTEX content of most straight-run gasolines does not total more than 10 to 12 percent. The straight separation of aromatics from crude oil is not recommended because of their low concentrations which result in low yields: some crude oils contain up to 6 percent aromatics.2 Aromatics are also extracted during the naphtha steam cracking process. The cracker feed contains between 10 and 25% of aromatics and their removal allows the efficiency of the process to be enhanced.However, the separation and production of aromatics with high-purity is a difficult task because of the close boiling points of the different hydrocarbons involved and the several combinations of azeotropes that may occur. Commercial separation methods are (i) liquid–liquid extractions suitable for the range of (20 to 65) wt% of aromatics, (ii) extractive distillation for aromatic concentrations between 65 and 90 wt%, and (iii) azeotropic distillation for higher aromatic contents (>90 wt%). Nevertheless, to date, there is no feasible process for the separation of aromatics from aliphatics with aromatic contents below 20 wt% in the feed mixture.3
The liquid extraction process is considered by many researchers as the most promising process for aromatic/aliphatic separation when the aromatic content does not exceed 20%. The main advantage of the liquid–liquid extraction process is the low energy consumption due to its mild operating conditions. In addition, during this process the chemical structure and the physical properties of the species involved are not affected. However, the performance of the extraction process strongly depends on the choice of solvent used. An ideal solvent should possess high selectivity towards the solute, a high distribution ratio for economic feed to solvent ratio and a high performance index. Besides, for practical reasons, the solvent should also have favourable and industrially feasible thermophysical and transport properties such as low viscosity and high thermal stability. Moreover, it should be environmentally benign, highly recoverable and available on a large scale at an affordable cost. Nowadays, the conventional solvents used for this purpose are organic compounds such as sulfolane, ethylene glycols, furfuryl alcohol, N-methylpyrrolidone (NMP), and N-formylmorpholine (NFM).4 But unfortunately most of these solvents are toxic, flammable, volatile and/or not highly recoverable. Therefore, alternative green solvents which can offer similar or better performance are desirable.
In recent years, ionic liquids (ILs) have been reported as potential alternatives to organic solvents for the liquid extraction of aromatics from aliphatic hydrocarbons.4–7 Reports on the ILs used include ammonium-based,4 pyridinium-based, and imidazolium-based ILs.5,8,9 ILs are considered as attractive solvents because of their negligible vapour pressure. However, there is still a challenge for large-scale applications of ionic liquids in industry due to their complicated synthesis process and expensive raw chemicals, in addition to their unknown toxicity.10 On the other hand, a new generation of solvents namely Deep Eutectic Solvents (DESs) has emerged as a low-cost alternative to ILs. A DES is a mixture of two or more compounds that form a eutectic with a melting point lower than that of each individual component. Depression of the freezing point is related to the strength of interaction between the components of the mixture. A hydrogen bond donor (HBD) or complexing agent (CA) can be combined with organic salts or metal halides to give a DES. The salt normally used for the synthesis of DESs consists of a large quaternary ammonium or phosphonium cation, combined with a halide anion. When the salt is combined with a HBD or a CA at a certain ratio, the HBD or CA will form a complex with the halide anion, thus lowering the freezing point of the produced eutectic mixture far from that of its individual constituents.11 An example of a common DES is the combination of choline chloride with urea, which forms a eutectic mixture when 1 mole of choline chloride is mixed with 2 moles of urea.12 In a molecular dynamic study performed by Sun et al.,13 the interaction energies of the cation and anion in the salt of a DES (choline chloride + urea) was compared before and after mixing with a complexing agent (urea). It was found that the addition of a complexing agent reduces the interaction energy between the cation and anion of the salt, and there is an increase in the interaction energy between anion and the complexing agent. This results in a modest interaction energy between cation–anion, cation–urea and anion–urea, which causes a lower melting point of the eutectic mixture. DESs have physico-chemical properties comparable to ionic liquids, especially the negligible vapour pressure which is characteristic of its non-volatility. Additionally, DESs have several advantages compared to traditional ionic liquids which include: (1) simple to synthesize; the materials can be easily mixed and ready to be used without further purification, (2) less expensive, and (3) DESs can be biodegradable and non-toxic based on the wise choice of their constituents.14,15 Hence, DESs are seen as potential candidates to be used as a replacement for ionic liquids.
The application of DESs for the separation of aromatics from aliphatics has been reported by Kareem et al. where phosphonium-based DESs were used for the separation of toluene from n-heptane16 and for the separation of benzene from hexane.17 More recently, the same group also reported the use of a different phosphonium-based DES for the separation of toluene from n-heptane.18 In their work, Kareem et al. reported the performance of the DES to be at par or even better than conventional organic solvents and ionic liquids. More recently, Li et al.19 reported the use of various ammonium-based DESs for extractive desulphurization, where the DESs used in their work were reported to have a very high efficiency for removal of benzothiophene from model oil. To date, these are the only reports on the use of DESs for the extraction of aromatic from aliphatic hydrocarbons. Acknowledging the importance and potential of DESs for the separation of aromatics from aliphatics, it is worthwhile to study the LLE of ternary mixtures of (DESs + aromatic hydrocarbon + alkanes). In this work, the performance of an ammonium-based DES to separate aromatics from aliphatics is reported. We focus our attention on BTEX aromatics while n-octane is chosen to be the representative compound of aliphatics. The DES used in this work is a combination of tetrabutylammonium bromide salt with sulfolane playing the role of the complexing agent. Sulfolane is known for its high distribution ratio and selectivity for the separation of aromatics.20,21 The LLE measurements for the ternary system (aromatic hydrocarbon + n-octane + DES) are reported at 25 °C. The aromatic compounds studied are benzene, toluene, ethyl benzene and m-xylene (BTEX). The experimental results were used to estimate the binary interaction parameters of the Non-Random Two-Liquid (NRTL) model. In addition, the performance of the COSMO-RS model to qualitatively predict a ternary LLE diagram with the DES is also evaluated. Based on the COSMO-RS model, the σ-profiles of the species involved are used to explain the behaviour of the ternary mixture. To the best of our knowledge, no work on the use of ammonium-based DESs for the separation of aromatics has been reported, and this is the first time that COSMO-RS has been used for the qualitative prediction of ternary LLE phase diagram for systems with DES.
Experimental
Chemicals
Pure grade compounds ethyl benzene and n-octane were purchased from Winlab (England), toluene was purchased from Merck (Germany), benzene was purchased from BDH (England), m-xylene, tetrabutylammonium bromide, and sulfolane were purchased from Acros Organics (Belgium). All chemicals were of high purity (>99%) and used without any further purification. The DES was prepared according to the method described by Abbott et al.12 The salt tetrabutylammonium bromide was mixed with sulfolane with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 in screw-capped bottles. The bottles were then stirred in an incubating-shaker at a temperature of 100 °C and a rotational speed of 200 rpm until a clear liquid was formed.
4 in screw-capped bottles. The bottles were then stirred in an incubating-shaker at a temperature of 100 °C and a rotational speed of 200 rpm until a clear liquid was formed.
Mixtures of aromatics and n-octane were prepared for nine aromatic concentrations (10, 15, 20, 30, 40, 50, 60, 70 and 80 wt%) by mixing weighed amounts of pure aromatic and pure n-octane using an analytical balance (±0.0001 g). The feed was then mixed with the DES in a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Each set of experiments was conducted at 25 °C. The vials were placed in an incubator shaker with temperature (±0.1 °C) and speed control. The shaking time was six hours followed by a settling time of about 12 hours to guarantee that the equilibrium state was completely attained.4 Samples were taken from the top and bottom layers and analyzed using a HPLC.
1. Each set of experiments was conducted at 25 °C. The vials were placed in an incubator shaker with temperature (±0.1 °C) and speed control. The shaking time was six hours followed by a settling time of about 12 hours to guarantee that the equilibrium state was completely attained.4 Samples were taken from the top and bottom layers and analyzed using a HPLC.
Analysis
Samples from the top and bottom layers were withdrawn using a syringe and then diluted using 2-propanol. The samples were analyzed using a HPLC Agilent 1100 series with a zorbax eclipse xdb-c8 column. The temperature of the column oven was set to 30 °C. The mobile phase was acetonitrile and distilled water with a volume ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The flow rate of the mobile phase was 1.4 ml min−1 with a pressure of 120 bars. The uncertainty in the reported concentrations was estimated to be ±0.001 wt% (10 ppm).
1. The flow rate of the mobile phase was 1.4 ml min−1 with a pressure of 120 bars. The uncertainty in the reported concentrations was estimated to be ±0.001 wt% (10 ppm).
Results and discussion
Tetrabutylammonium bromide salt was used in this study because it is available commercially with a moderate price. In addition, it was found that the melting points and viscosities of DESs based on this salt are much less than other DESs. Sulfolane was used as the complexing agent because it is the most commercially used solvent for the separation of aromatics. Hence, the current process in use will not require a dramatic change when examined with this DES.In order to investigate the feasibility for potential application of this DES to perform extraction of aromatic hydrocarbons from a mixture with n-octane the parameters distribution ratio (D) and selectivity (S) were calculated from experimental data.
These parameters are defined by the following expressions:
| D = xIIaro/xIaro | (1) |
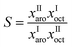 | (2) |
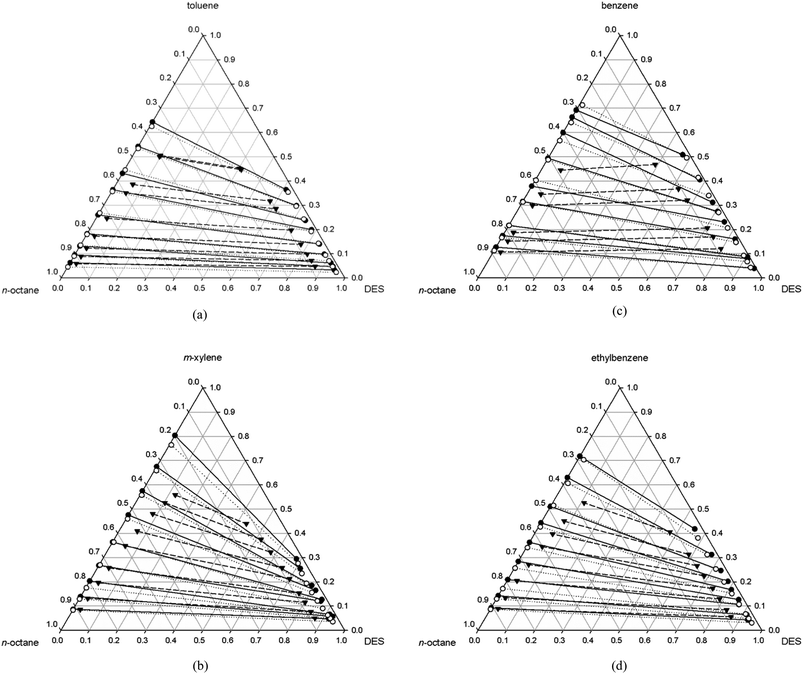
The measured compositions at equilibrium in both liquid phases for each DES are reported in Tables 1–4 and also plotted in the ternary diagrams given in Fig. 1a–d.
| % Aro | n-Octane-rich phase | DES-rich phase | D | S | ||||
|---|---|---|---|---|---|---|---|---|
| x1 | x2 | x3 | x1 | x2 | x3 | |||
| 5 | 0.063 | 0.937 | 0.000 | 0.033 | 0.020 | 0.947 | 0.53 | 24.5 |
| 10 | 0.095 | 0.905 | 0.000 | 0.047 | 0.017 | 0.935 | 0.50 | 25.7 |
| 15 | 0.132 | 0.868 | 0.000 | 0.062 | 0.018 | 0.921 | 0.47 | 23.3 |
| 20 | 0.183 | 0.817 | 0.000 | 0.098 | 0.023 | 0.879 | 0.54 | 19.4 |
| 30 | 0.260 | 0.740 | 0.000 | 0.140 | 0.019 | 0.841 | 0.54 | 20.9 |
| 40 | 0.364 | 0.636 | 0.000 | 0.199 | 0.018 | 0.783 | 0.55 | 19.4 |
| 50 | 0.432 | 0.568 | 0.000 | 0.237 | 0.020 | 0.742 | 0.55 | 15.3 |
| 60 | 0.543 | 0.457 | 0.000 | 0.299 | 0.021 | 0.680 | 0.55 | 12.0 |
| 70 | 0.643 | 0.357 | 0.000 | 0.364 | 0.024 | 0.611 | 0.57 | 8.3 |
| % Aro | n-Octane-rich phase | DES-rich phase | D | S | ||||
|---|---|---|---|---|---|---|---|---|
| x1 | x2 | x3 | x1 | x2 | x3 | |||
| 10 | 0.087 | 0.913 | 0.000 | 0.047 | 0.032 | 0.920 | 0.55 | 15.4 |
| 15 | 0.139 | 0.861 | 0.000 | 0.058 | 0.013 | 0.929 | 0.42 | 27.2 |
| 20 | 0.204 | 0.796 | 0.000 | 0.066 | 0.020 | 0.914 | 0.33 | 13.1 |
| 30 | 0.268 | 0.732 | 0.000 | 0.120 | 0.025 | 0.855 | 0.45 | 13.4 |
| 40 | 0.365 | 0.635 | 0.000 | 0.127 | 0.017 | 0.856 | 0.35 | 12.9 |
| 50 | 0.476 | 0.524 | 0.000 | 0.165 | 0.020 | 0.815 | 0.35 | 9.3 |
| 60 | 0.575 | 0.425 | 0.000 | 0.186 | 0.023 | 0.791 | 0.32 | 5.9 |
| 70 | 0.675 | 0.325 | 0.000 | 0.255 | 0.029 | 0.716 | 0.38 | 4.2 |
| 80 | 0.804 | 0.196 | 0.000 | 0.295 | 0.023 | 0.682 | 0.37 | 3.2 |
| % Aro | n-Octane-rich phase | DES-rich phase | D | S | ||||
|---|---|---|---|---|---|---|---|---|
| x1 | x2 | x3 | x1 | x2 | x3 | |||
| 10 | 0.127 | 0.873 | 0.000 | 0.040 | 0.007 | 0.953 | 0.31 | 39.7 |
| 15 | 0.174 | 0.826 | 0.000 | 0.080 | 0.010 | 0.910 | 0.46 | 39.1 |
| 20 | 0.217 | 0.783 | 0.000 | 0.087 | 0.007 | 0.906 | 0.40 | 46.4 |
| 30 | 0.316 | 0.684 | 0.000 | 0.160 | 0.014 | 0.826 | 0.51 | 25.1 |
| 40 | 0.379 | 0.621 | 0.000 | 0.231 | 0.016 | 0.752 | 0.61 | 23.0 |
| 50 | 0.494 | 0.506 | 0.000 | 0.274 | 0.019 | 0.707 | 0.56 | 15.2 |
| 60 | 0.599 | 0.401 | 0.000 | 0.312 | 0.017 | 0.671 | 0.52 | 12.1 |
| 70 | 0.663 | 0.337 | 0.000 | 0.406 | 0.016 | 0.579 | 0.61 | 13.1 |
| 80 | 0.692 | 0.308 | 0.000 | 0.509 | 0.025 | 0.467 | 0.74 | 9.2 |
| % Aro | n-Octane-rich phase | DES-rich phase | D | S | ||||
|---|---|---|---|---|---|---|---|---|
| x1 | x2 | x3 | x1 | x2 | x3 | |||
| 10 | 0.093 | 0.907 | 0.000 | 0.043 | 0.028 | 0.929 | 0.47 | 15.3 |
| 15 | 0.143 | 0.857 | 0.000 | 0.064 | 0.021 | 0.915 | 0.45 | 18.7 |
| 20 | 0.210 | 0.790 | 0.000 | 0.106 | 0.028 | 0.866 | 0.51 | 14.4 |
| 30 | 0.284 | 0.716 | 0.000 | 0.127 | 0.018 | 0.855 | 0.45 | 17.7 |
| 40 | 0.363 | 0.637 | 0.000 | 0.168 | 0.023 | 0.809 | 0.46 | 12.6 |
| 50 | 0.444 | 0.557 | 0.000 | 0.202 | 0.018 | 0.780 | 0.46 | 13.8 |
| 60 | 0.512 | 0.488 | 0.000 | 0.247 | 0.021 | 0.732 | 0.48 | 11.1 |
| 70 | 0.630 | 0.370 | 0.000 | 0.313 | 0.021 | 0.667 | 0.50 | 8.8 |
| 80 | 0.718 | 0.282 | 0.000 | 0.418 | 0.027 | 0.555 | 0.58 | 6.0 |
Consistency of the liquid–liquid equilibrium data
The reliability of the experimental results have been ascertained by using Othmer–Tobias22 and Hand23 correlations given respectively by:
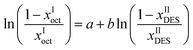 | (3) |
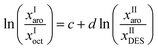 | (4) |
The linearity of each plot indicates the degree of consistency of the data. The parameters of the Othmer–Tobias and Hand correlation are given in Table 5. The regression coefficients R2 are very close to unity which indicates the degree of consistency of our experimental data.
| Aromatics | Othmer–Tobias | Hand | ||||
|---|---|---|---|---|---|---|
| a | b | R2 | c | d | R2 | |
| Toluene | 1.665 | 1.328 | 0.997 | 1.466 | 1.124 | 0.997 |
| m-Xylene | 3.296 | 1.841 | 0.971 | 3.103 | 1.597 | 0.985 |
| Benzene | 1.111 | 0.964 | 0.985 | 1.127 | 0.922 | 0.985 |
| Ethylbenzene | 1.907 | 1.390 | 0.993 | 1.190 | 1.191 | 0.997 |
Selectivity and distribution ratio
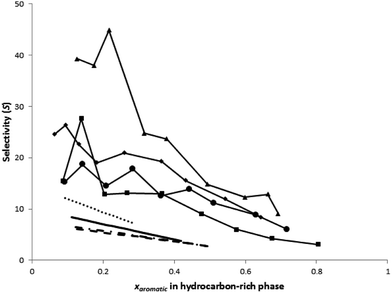
Fig. 2 shows the aromatic selectivity as a function of its concentration in the raffinate. It could be noticed that the selectivity of the aromatic compound decreases with the increase of the concentration of the aromatic in the raffinate, in most cases.This is in agreement with results reported earlier in the literature for other systems using different DESs.16–18 For the system m-xylene/n-octane/DES it can be noticed that the selectivity for aromatics is less than that in toluene/n-octane/DES. This could be attributed to the fact that m-xylene is less polar than toluene. The same argument applies to the other studied systems where selectivity decreases with a decrease of the aromatic compound under the same conditions.
Most importantly, it can be noticed that the sulfolane, and hence the DES, was not found in the raffinate layer. This may be attributed to the hydrogen bonding between the salt and the sulfolane. This is very important from an industrial point of view. Especially when we know that when pure sulfolane is used as a solvent for aromatics extraction its concentration in the raffinate layer may reach 20 wt%; causing solvent loss and requiring additional separation steps.
A comparison between the values of selectivity and distribution ratio for the studied DES and those reported in the literature for organic solvents and different types of ILs is shown in Table 6. The comparison shows that the selectivity of the used DES is less than that for other solvents, while the distribution ratio for this DES is higher than that for other solvents. In spite of this, DESs are advantageous because they can be easily prepared in high purity at low cost; thus being cheaper than ionic liquids.
| System | Solvent | T (K) | S | D | Ref. |
|---|---|---|---|---|---|
| Toluene/n-octane | DES | 298 | 25.7 | 0.50 | This work |
| [EMPY][EtSO4] | 298 | 55.2 | 0.25 | 24 | |
| [EMIM][EtSO4] | 298 | 68.1 | 0.23 | 25 | |
| Sulfolane | 298 | 44.1 | 0.37 | 26 | |
| m-Xylene/n-octane | DES | 298 | 27.2 | 0.42 | This work |
| C2NTf2 | 298 | 27.0 | 0.30 | 4 | |
| [MEBUPY]BF4 | 313 | 42.6 | 0.36 | 27 | |
| [MEBUPY]BF4 | 348 | 39.2 | 0.32 | 27 | |
| [EMIM][NTf2] | 298 | 31.3 | 0.47 | 28 | |
| Sulfolane | 298 | 34.5 | 0.29 | 26 | |
| Benzene/n-octane | DES | 298 | 46.4 | 0.40 | This work |
| [EMIM][ESO4] | 298 | 155.7 | 0.52 | 29 | |
| [BMIM][NTf2] | 298 | 38.7 | 1.62 | 30 | |
| [EMPY][ESO4] | 298 | 160.0 | 0.84 | 31 | |
| [EPY][EtSO4] | 298 | 89.8 | 0.44 | 32 | |
| Sulfolane | 298 | 48.9 | 0.65 | 26 | |
| Ethylbenzene/n-octane | DES | 298 | 18.7 | 0.45 | This work |
| [EMIM][NTf2] | 298 | 30.4 | 0.48 | 33 | |
| [MEBUPY][BF4] | 313 | 64.3 | 0.50 | 27 | |
| [MEBUPY][BF4] | 348 | 51.9 | 0.44 | 27 | |
| [OMIM][SCN] | 298 | 6.38 | 0.67 | 9 | |
| Propylene carbonate | 298 | 7.88 | 0.38 | 34 |
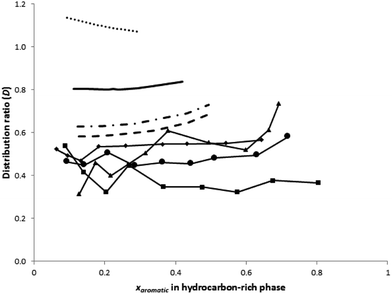
Fig. 3 reports the distribution ratio of BTEX aromatics as a function of their concentration in the raffinate phase. The values of the solute distribution ratio show a slight increase with the reduction of aromatic mass fraction in the feed. Furthermore, the distribution ratio values are less than unity for all aromatics.
This means that a large amount of the DES should be used as a solvent for the separation. However, as the DES can be recovered and reused, this problem should not be considered as a serious disadvantage.
NRTL modelling
When performing a liquid–liquid equilibrium calculation, phase compositions are obtained by solving an isothermal liquid–liquid flash. This flash consists of the following system of equations:Material balance:
| xi − (1 − ω)xL1i − ωxL2i = 0, i = 1, Nc | (5) |
Equilibrium equation:
| xL1iγL1i − xL2iγL2i = 0, i = 1, Nc | (6) |
Equation of summation
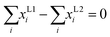 | (7) |
Where, ω is the liquid–liquid splitting ratio, xi is the composition of component i in the mixture, xL1i is the composition of component i in the liquid phase L1, xL2i is the composition of component i in the liquid phase L2, and γL1i and γL2i are the activity coefficients of component i in the liquid phase L1 and L2 respectively. NC is the number of constituents.
The activity coefficients, expressing the non-ideality of the liquid phase, were estimated using the NRTL thermodynamic model.35
The parameters of this model are (i) the binary interaction parameters (τij, τji) and (ii) the non-randomness parameter (αij). In this work, the value of the third non-randomness parameter, αij, was fixed to 0.20 while the interaction parameters τij and τji were estimated from “6m” experimental data points (where m represents the number of tie-lines) by minimizing the Root Mean Square Deviation (RMSD) between the experimental and calculated data points at each tie-line as follows:
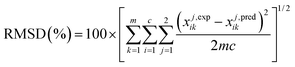 | (8) |
The model development was achieved within a Simulis® environment, a thermo physical properties calculation server provided by ProSim36 and available as an MS-Excel add-in. The RMSD for each ternary system is presented in Table 7.
| System | RMSD (%) | |
|---|---|---|
| NRTL | COSMO-RS | |
| DES + toluene + n-octane | 0.75 | 9.65 |
| DES + m-xylene + n-octane | 1.44 | 7.33 |
| DES + benzene + n-octane | 1.43 | 12.70 |
| DES + ethylbenzene + n-octane | 1.76 | 6.50 |
Table 8 shows the values of the binary interaction parameters obtained using the NRTL model with each ternary system. As can be seen, the interaction between n-octane and the DES was considered independent of the aromatic compound.
| i | j | τij | τji |
|---|---|---|---|
| n-Octane | DES | 1763.9 | 705.8 |
| Benzene | n-Octane | 408.8 | −717.6 |
| Benzene | DES | 1296.6 | −618.4 |
| Toluene | n-Octane | 98.3 | −163.3 |
| Toluene | DES | 1402.7 | −403.2 |
| Ethyl benzene | n-Octane | −356.3 | 316.2 |
| Ethyl benzene | DES | 1247.8 | −305.7 |
| m-Xylene | n-Octane | −614.9 | 1083.8 |
| m-Xylene | DES | 1608.2 | −183.5 |
The previous Fig. 1a–d show the ternary diagrams including the calculated compositions by NRTL correlation. It can be seen from these figures that the calculated compositions have good agreement with the experimental ones, and the tie-lines of both compositions coincide in most cases.
COSMO-RS model and computational details
The conductor-like screening model for a real solvent (COSMO-RS) is based on a unimolecular quantum chemical calculation which is combined with statistical thermodynamic approaches to describe thermodynamic behaviour of pure compounds and mixtures of compounds.37 COSMO-RS provides a link between quantum chemistry and chemical engineering thermodynamics at a molecular level without requiring any experimental data on the studied system. COSMO-RS can be applied to predict various thermodynamic properties and behavior of a mixture, including binary and ternary liquid–liquid equilibrium phase diagrams. Expressions for the chemical potential and activity coefficient were derived by Klamt and co-workers.38 The interaction between species involved can be derived from the σ-profile and σ-potential. The σ-profile represents the probability distribution of finding a surface segment with a specific screening charge density. It should be noted that in a σ-profile, negative σ represents positive polarities, and vice versa. Meanwhile, the σ-potential describes the affinity of a solvent for a molecular surface of polarity σ. A lower μ(σ) value at a particular σ implies better affinity for that polarity, and vice versa.The first step in COSMO-RS is to generate a cosmo file from the optimized geometry of each species involved. Geometry optimization was performed for the cation, anion, complexing agent, aliphatic and aromatic compounds studied in this work. Initial structures for each compound were drawn and the job was executed to perform geometry optimization at the Hartree–Fock level and 6-31G* basis set. The calculation of geometry optimization at the Hartree–Fock level gives more meaningful and accurate values,39 while the 6-31G* basis set accounts for the polarization effect of the species in the complexes. From the optimized geometry for each individual species, a single point calculation was performed with activation of cosmo file generation using density functional theory with a Becke–Perdew functional and triple zeta valence potential (TZVP) basis set. All of these jobs were performed with Turbomole Software Package.40 Thereafter, the cosmo files were imported into COSMOthermX software package with the parameterization file BP_TZVP_C30_1301.ctd.41 The σ-profiles, σ-potentials, and the ternary liquid–liquid equilibrium phase diagram were retrieved from COSMOthermX software.
COSMO-RS has been widely used for the prediction of binary and ternary LLE diagrams for systems with ionic liquids. These include mixtures of ionic liquids with aromatics and aliphatics.42–45 However, to the best of our knowledge, there is no published report on the use of COSMO-RS to predict phase equilibria for systems with a DES. Hence, in this work, the performance of COSMO-RS to predict ternary LLE for systems with a DES is evaluated against the reported experimental data. Besides, COSMO-RS is also used to understand the interaction between the DES and aromatic and aliphatic compounds based on σ-profiles.
COSMO-RS representation of the DES
There are three approaches to describe ionic liquids in COSMO-RS: (i) the electro-neutral approach, (ii) the ion pair approach and (iii) the meta-file approach. The first approach is considered to be the closest representation of ionic liquids in COSMO-RS because it describes the two ions as separate species in a liquid mixture.41 Hence, this approach was also adopted in this work to describe the DES in COSMO-RS as it is believed that in liquid form, the constituents of the DES are three distinct species, i.e. cation, anion and CA. Consider a DES with a salt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA ratio of 1
CA ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n. In an actual experimental situation, this means that the DES consists of 1 mole of cation, 1 mole of anion and n moles of CA. Thus, compared to the representation of the ionic liquid as 1
n. In an actual experimental situation, this means that the DES consists of 1 mole of cation, 1 mole of anion and n moles of CA. Thus, compared to the representation of the ionic liquid as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for its cation
1 for its cation![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anion ratio, the DES is represented as 1
anion ratio, the DES is represented as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n for its cation
n for its cation![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anion
anion![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA ratio. In this study, the ratio of cation
CA ratio. In this study, the ratio of cation![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anion
anion![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA is 1
CA is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.
4.
However, by using approach (i) to represent the DES in COSMO-RS, the mole fractions obtained by COSMO-RS calculations need to be converted to reflect the actual experimental definition of the mole fraction.
The experimental definition of the mole fraction of a solute in a mixture of solute, carrier and DES is given by:
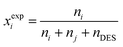 | (9) |
On the other hand, the definition of mole fraction in COSMO-RS by using the electroneutral approach is given by:
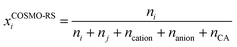 | (10) |
From eqn (9) and (10), we can obtain the conversion from the calculated mole fraction in COSMOthermX (xCOSMO-RSi) to the laboratory framework (xLABi):
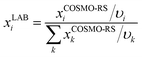 | (11) |
Prediction of LLE with COSMO-RS
The RMSD for each ternary system is presented in Table 6. All systems except for the system with benzene demonstrate RMSD < 10%. This is good considering COSMO-RS is an a priori method which predicts the points solely based on the chemical structure of the species involved. Nevertheless, it can be observed that quantitatively, the predicted tie-lines are not in good agreement with experimental results. However, the predicted LLE did reflect a qualitative trend as shown by the experimental data, having negative slopes for the phase diagram (with the exception of the system benzene–toluene–DES). While in the experimental results, the concentration of DES in the hydrocarbon-rich phase is found to be zero (due to the absence of a sulfolane compound in the raffinate phase), COSMO-RS predicts a small amount of DES to exist in the raffinate phase. From the diagrams, it is also observed that COSMO-RS provides a better prediction of the tie-lines at low aromatic concentrations, which is sufficient for the objective of this work, i.e. to target separation of aromatics at low concentrations.Besides the ternary diagrams, evaluation of COSMO-RS for use in ternary systems with a DES can also be performed by comparing the predicted distribution ratios and selectivity against those obtained from the experiment. From Fig. 2, it can be seen that COSMO-RS overestimates the distribution ratio of aromatics in the two phases. However, COSMO-RS underestimates the selectivity of aromatics as shown in Fig. 2, where the reported values of predicted selectivity are much lower than those obtained from the experiment. Because the strength of the COSMO-RS prediction lies in its relativity and not its absolute values, it is more important and meaningful to observe whether the prediction of distribution ratios and selectivity give the correct trend as given by the experimental results. From Fig. 3, the distribution ratio predicted by COSMO-RS for the separation of aromatics from toluene using a DES is in the order of benzene > toluene > ethylbenzene > m-xylene. This is in accordance with the experimental results, where the highest distribution ratios are reported for benzene, followed by toluene, ethylbenzene and m-xylene. Comparison of the predicted selectivity can be done in a similar fashion. From Fig. 3, COSMO-RS reported the predicted selectivity in the order of benzene > toluene > ethylbenzene > m-xylene, which is again in the same order as the calculated selectivity based on experimental values. Therefore, although COSMO-RS does not provide satisfactory prediction values for the LLE phase diagram, selectivity and distribution ratio, it is useful as a screening tool to evaluate the performance of a DES for the separation of aromatics based on selectivity and distribution ratio.
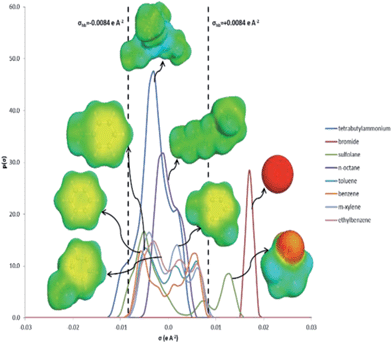
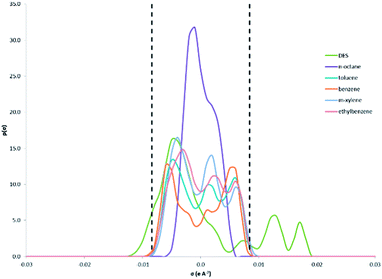
The σ-profiles of all species involved are shown in Fig. 4. As mentioned previously, negative σ values represent positive polarities, and vice versa. The cation tetrabutylammonium shows a narrow peak at slightly negative σ values. In COSMO-RS, a narrow σ-profile indicates less polar character compared to species with a broad σ-profile. The narrow peak of tetrabutylammonium also means that it has a concentrated positive charge, which revolves around the N atom in the structure. This cation is less polar due to the presence of four butyl chains surrounding the N atom, and shows a slight peak at σ = +0.002 e Å−2.
The anion Br− shows a peak at σ = +0.017 e Å−2 which is solely due to its negative charge. The σ-profile of the complexing agent sulfolane is broad indicating its polar characteristics, with peaks at σ = −0.005 e Å−2 due to CH atoms and σ = −0.013 e Å−2 due to S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds. The bromide anion and sulfolane both show peaks at σ >+0.0084 e Å−2, which indicate the presence of a hydrogen bond acceptor in each species. The σ-profiles of aromatic compounds (toluene, m-xylene, benzene and ethylbenzene) look similar in shape, but benzene demonstrates a more symmetric σ-profile with peaks at σ = −0.006 e Å−2 due to the polarized hydrogens and at σ = +0.006 e Å−2 due to the π-face of the benzene ring. The σ-profile of n-octane is that of a typical alkane, where the peaks are due to carbon at the positive σ values, and due to hydrogen at the negative σ values. Fig. 5 shows σ-profiles similar to Fig. 4, but in this case the DES is taken as a mixture rather than separate compounds. As observed, the σ-profile of the aromatic compounds is complementary with that of the DES, which thus explains why aromatics are soluble in the DES and n-octane is not. Aliphatic compounds have a very small electrostatic field which is usually approximated to zero due to the saturated nature of its carbon–carbon and carbon-hydrogen bonds.13 If n-octane is mixed with the DES, n-octane needs to interact with the less polar surface segments in the DES, which are not available in the DES as the polar surface segments. Meanwhile, aromatic compounds have electron density both above and below the plane of the ring.13 Hence, as demonstrated by the σ-profile, the aromatic compounds have polar surface segments which can interact with the many polar surface segments in DES. Therefore, a reasonable explanation as to why the DES is able to extract aromatic compounds from aliphatics may be due to electrostatic interactions between the solute (aromatic compound) and solvent (DES).
O bonds. The bromide anion and sulfolane both show peaks at σ >+0.0084 e Å−2, which indicate the presence of a hydrogen bond acceptor in each species. The σ-profiles of aromatic compounds (toluene, m-xylene, benzene and ethylbenzene) look similar in shape, but benzene demonstrates a more symmetric σ-profile with peaks at σ = −0.006 e Å−2 due to the polarized hydrogens and at σ = +0.006 e Å−2 due to the π-face of the benzene ring. The σ-profile of n-octane is that of a typical alkane, where the peaks are due to carbon at the positive σ values, and due to hydrogen at the negative σ values. Fig. 5 shows σ-profiles similar to Fig. 4, but in this case the DES is taken as a mixture rather than separate compounds. As observed, the σ-profile of the aromatic compounds is complementary with that of the DES, which thus explains why aromatics are soluble in the DES and n-octane is not. Aliphatic compounds have a very small electrostatic field which is usually approximated to zero due to the saturated nature of its carbon–carbon and carbon-hydrogen bonds.13 If n-octane is mixed with the DES, n-octane needs to interact with the less polar surface segments in the DES, which are not available in the DES as the polar surface segments. Meanwhile, aromatic compounds have electron density both above and below the plane of the ring.13 Hence, as demonstrated by the σ-profile, the aromatic compounds have polar surface segments which can interact with the many polar surface segments in DES. Therefore, a reasonable explanation as to why the DES is able to extract aromatic compounds from aliphatics may be due to electrostatic interactions between the solute (aromatic compound) and solvent (DES).
The order of selectivity and distribution ratio of aromatics with the DES (i.e. benzene > toluene > m-xylene > ethyl benzene) can also be explained from their σ-profiles. Benzene has more polar characteristics than other aromatics in this study. As can be seen, the peaks of aromatic σ-profiles at the negative side become closer to zero from benzene, toluene, m-xylene and ethyl benzene, albeit a small difference between them. The addition of a methyl and/or ethyl group to the benzene ring in toluene, m-xylene and ethyl benzene reduces the charge concentration in the π-face of the benzene ring and the charge becomes more distributed around the methyl or ethyl group. This reduction of charge concentration results in less strength in the electrostatic interaction between the solute and solvent. The more CH groups added to the benzene ring, the more significant the reduction in the electrostatic interaction becomes. Ethyl benzene, having the most CH groups added to the benzene ring, thus has the lowest selectivity and distribution ratio.
Conclusion
The separation of aromatic from aliphatic hydrocarbons is a challenging process. In this work, deep eutectic solvents synthesized from an ammonium salt combined with sulfolane were used for the liquid–liquid extraction of BTEX aromatics from n-octane. Sulfolane was selected as the complexing agent due to its high selectivity for aromatics. The ability of the synthesized DES to selectively extract aromatics from a mixture of aromatic and aliphatic compounds was proven. Sulfolane was not found in the raffinate phase. This remarkable finding will undeniably facilitate the separation protocol and hence reduce the cost of the separation process. The performance of COSMO-RS to predict ternary LLE diagrams for systems with DES was evaluated and it was observed that the prediction by COSMO-RS only agrees qualitatively with the experimental tie-lines and the trend for distribution ratio and selectivity. Nevertheless, σ-profiles of the species involved are useful to explain the interaction between the DES with the aromatic compounds. Moreover, the experimental data were satisfactorily correlated using the NRTL model, showing that this classical model can easily be adopted with systems including deep eutectic solvents.Nomenclature
| [EMPY][EtSO4] | 1-Ethyl-3-methylpyridinium ethylsulfate |
| [EMIM][EtSO4] | 1-Ethyl-3-methylimidazolium ethylsulfate |
| C2NTf2 | Ethyl(2-hydroxyethyl)dimethylammonium bis(trifluoromethyl)sulfonylimide |
| [MEBUPY]BF4 | 4-Methyl-N-butylpyridinium tetrafluoroborate |
| [EMIM][NTf2] | 1-Ethyl-3-methylimidazolium bis(trifluoromethyl)sulfonylimide |
| [BMIM][NTf2] | 1-Butyl-3-methylimidazolium bis(trifluoromethyl)sulfonylimide |
| [EPY][EtSO4] | 1-Ethylpyridinium ethylsulfate |
| [OMIM][SCN] | 1-Octyl-3-methylimidazolium dicyanamide. |
Acknowledgements
This research was funded by the Deanship of Scientific Research at King Saud University through the group project number RGP-VPP-108 in collaboration with the University of Malaya Centre for Ionic Liquids (UMCiL) through the HIR grant number HIR-MOHE (D000003-16001).Notes and references
- M. A. Rodríguez and J. Ancheyta, Fuel, 2011, 90, 3492–3508 CrossRef PubMed.
- F. W. Melpolder, R. A. Brown, W. S. Young and C. E. Headington, Ind. Eng. Chem., 1952, 44, 1142–1146 CrossRef CAS.
- K. a. A. Weissermel and H. J. Arpe, Industrial Organic Chemistry, Wiley-VCH, 2003 Search PubMed.
- U. Domańska, A. Pobudkowska and M. Królikowski, Fluid Phase Equilib., 2007, 259, 173–179 CrossRef PubMed.
- N. Calvar, I. Domínguez, E. Gómez and Á. Domínguez, Chem. Eng. J., 2011, 175, 213–221 CrossRef CAS PubMed.
- U. K. Ravilla and T. Banerjee, Fluid Phase Equilib., 2012, 324, 17–27 CrossRef CAS PubMed.
- J. Zhang, C. Huang, B. Chen, P. Ren and Z. Lei, Energy Fuels, 2007, 21, 1724–1730 CrossRef CAS.
- J. Garcia, M. Larriba, J. S. Torrecilla and F. Rodriguez, Chemical Engineering Transactions, 2011, 24, 805–810 Search PubMed.
- M. R. Heidari, B. Mokhtarani, N. Seghatoleslami, A. Sharifi and M. Mirzaei, J. Chem. Thermodyn., 2012, 54, 310–315 CrossRef CAS PubMed.
- G. Wytze Meindersma, A. Podt and A. B. de Haan, Fuel Process. Technol., 2005, 87, 59–70 CrossRef CAS PubMed.
- Q. Zhang, K. De Oliveira Vigier, S. Royer and F. Jerome, Chem. Soc. Rev., 2012, 41, 7108–7146 RSC.
- A. P. Abbott, D. Boothby, G. Capper, D. L. Davies and R. K. Rasheed, J. Am. Chem. Soc., 2004, 126, 9142–9147 CrossRef CAS PubMed.
- H. Sun, Y. Li, X. Wu and G. Li, J. Mol. Model., 2013, 19, 2433–2441 CrossRef CAS PubMed.
- Y. Dai, J. van Spronsen, G.-J. Witkamp, R. Verpoorte and Y. H. Choi, Anal. Chim. Acta, 2013, 766, 61–68 CrossRef CAS PubMed.
- H.-R. Jhong, D. S.-H. Wong, C.-C. Wan, Y.-Y. Wang and T.-C. Wei, Electrochem. Commun., 2009, 11, 209–211 CrossRef CAS PubMed.
- M. A. Kareem, F. S. Mjalli, M. A. Hashim, M. K. O. Hadj-Kali, F. S. G. Bagh and I. M. Alnashef, Fluid Phase Equilib., 2012, 333, 47–54 CrossRef CAS PubMed.
- M. A. Kareem, F. S. Mjalli, M. A. Hashim and I. M. AlNashef, Fluid Phase Equilib., 2012, 314, 52–59 CrossRef CAS PubMed.
- M. A. Kareem, F. S. Mjalli, M. A. Hashim, M. K. O. Hadj-Kali, F. S. Ghareh Bagh and I. M. Alnashef, J. Chem. Thermodyn., 2013, 65, 138–149 CrossRef CAS PubMed.
- C. Li, D. Li, S. Zou, Z. Li, J. Yin, A. Wang, Y. Cui, Z. Yao and Q. Zhao, Green Chem., 2013, 15, 2793–2799 RSC.
- J. Mahmoudi and M. N. Lotfollahi, J. Chem. Thermodyn., 2010, 42, 466–471 CrossRef CAS PubMed.
- R. S. Santiago and M. Aznar, Fluid Phase Equilib., 2007, 253, 137–141 CrossRef CAS PubMed.
- D. F. Othmer and P. E. Tobias, Ind. Eng. Chem., 1942, 34, 690–692 CrossRef CAS.
- D. B. Hand, J. Phys. Chem., 1929, 34, 1961–2000 CrossRef.
- E. J. González, N. Calvar, B. González and Á. Domínguez, J. Chem. Thermodyn., 2010, 42, 752–757 CrossRef PubMed.
- E. J. González, N. Calvar, I. Domínguez and Á. Domínguez, J. Chem. Thermodyn., 2011, 43, 562–568 CrossRef PubMed.
- J. Chen, L.-P. Duan, J.-G. Mi, W.-Y. Fei and Z.-C. Li, Fluid Phase Equilib., 2000, 173, 109–119 CrossRef CAS.
- G. W. Meindersma, A. Podt and A. B. de Haan, J. Chem. Eng. Data, 2006, 51, 1814–1819 CrossRef CAS.
- A. Arce, M. J. Earle, H. Rodríguez, K. R. Seddon and A. Soto, Fluid Phase Equilib., 2010, 294, 180–186 CrossRef CAS PubMed.
- E. J. González, N. Calvar, E. Gómez and A. n. Domínguez, J. Chem. Eng. Data, 2010, 55, 3422–3427 CrossRef.
- I. Domínguez, E. J. González, R. González and A. n. Domínguez, J. Chem. Eng. Data, 2011, 56, 3376–3383 CrossRef.
- E. J. González, N. Calvar, E. Gómez and Á. Domínguez, J. Chem. Thermodyn., 2010, 42, 104–109 CrossRef PubMed.
- E. Gómez, I. Domínguez, B. a. González and A. n. Domínguez, J. Chem. Eng. Data, 2010, 55, 5169–5175 CrossRef.
- A. Arce, M. J. Earle, H. Rodriguez, K. R. Seddon and A. Soto, Green Chem., 2008, 10, 1294–1300 RSC.
- M. C. Annesini, F. Gironi, L. Marrelli and I. Kikic, J. Chem. Eng. Data, 1985, 30, 195–196 CrossRef CAS.
- H. Renon and J. M. Prausnitz, AIChE J., 1968, 14, 135–144 CrossRef CAS.
- PROSIM, http://www.prosim.net/.
- A. Klamt, J. Phys. Chem., 1995, 99, 2224–2235 CrossRef CAS.
- A. Klamt and F. Eckert, Fluid Phase Equilib., 2000, 172, 43–72 CrossRef CAS.
- H. Lin, D.-l. Wu, L. Liu and D.-z. Jia, J. Mol. Struct.: THEOCHEM, 2008, 850, 32–37 CrossRef CAS PubMed.
- TURBOMOLE, 6.4 edn, 2012.
- C. G. C. KG, Burscheider Str. 515, D-51381 Leverkusen, Germany, Version C30_1301 edn, 12 December, 2012.
- A. R. Ferreira, M. G. Freire, J. C. Ribeiro, F. M. Lopes, J. G. Crespo and J. A. P. Coutinho, Ind. Eng. Chem. Res., 2012, 51, 3483–3507 CrossRef CAS.
- N. R. Varma, A. Ramalingam and T. Banerjee, Chem. Eng. J., 2011, 166, 30–39 CrossRef CAS PubMed.
- A. R. Ferreira, M. G. Freire, J. C. Ribeiro, F. M. Lopes, J. o. G. Crespo and J. o. A. P. Coutinho, Ind. Eng. Chem. Res., 2011, 50, 5279–5294 CrossRef CAS.
- S. Potdar, R. Anantharaj and T. Banerjee, J. Chem. Eng. Data, 2012, 57, 1026–1035 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |