Platinum nanoparticles supported on ionic liquid-modified-silica gel: hydrogenation catalysts
Lucas Foppaa,
Jairton Duponta and
Carla W. Scheeren*b
aLaboratory of Physical Chemistry, School of Chemistry and Food, Universidade Federal do Rio Grande-FURG, Av. Itália Km 08, 96201-900 Rio Grande, RS, Brazil
bInstitute of Chemistry, Laboratory of Molecular Catalysis UFRGS, Av. Bento Gonçalves, 9500, 91501-970 B.O. Box 15003, Porto Alegre, RS, Brazil. E-mail: carlascheeren@gmail.com
First published on 25th March 2014
Abstract
Platinum nanoparticles (ca. 2.3 nm) dispersed in ionic liquids and functionalized ionic liquids were supported within a silica network by the sol–gel method. The effect of the sol–gel catalyst (acid or base) on the encapsulated ionic liquid and on the platinum content was studied, and the silica morphology, the texture of the support material, and the hydrogenation activity were investigated. The Pt(0) content in the resulting xerogels (ca. 0.2 wt% Pt/SiO2) was shown to be independent of the sol–gel process. The acidic conditions resulted in xerogels with larger pore diameters, which in turn might be responsible for the higher catalytic activity in hydrogenation of the alkenes and arenes obtained with the heterogeneous catalyst (Pt(0)/SiO2).
1. Introduction
Supported Pt catalysts are used in various industrial processes including hydrogenation, naphtha reforming, oxidation, automotive exhaust catalysts, and fuel cells.1–7 Pt/SiO2 is a classical model of Pt catalysts with SiO2 as the “inert” oxide support.8 SiO2 resists reduction and has low surface acidity, making it relatively inert compared to other oxide supports, such as TiO2 and Al2O3.9 These characteristics make Pt/SiO2 an ideal starting point for study of the catalytic role of Pt.10,11 It is well known that several steps in the catalyst preparation process strongly influence particle size in Pt/SiO2, including the support composition, metal salt, precursor deposition method, metal loading, pH, drying conditions, calcination temperature, and reduction temperature, among others.12,13The combination of an ionic liquid with a solid support material is emerging as a new alternative for the immobilization of transition metal catalyst precursors.14,15 Imidazolium ionic liquids (ILs) possess pre-organized structures mainly through hydrogen bonds which induce structural directionality.16 These IL structures can adapt or be adaptable to many species, as they provide hydrophobic or hydrophilic regions, and a high directional polarizability.17,18 This structural organization of ILs can be used as “entropic drivers” for spontaneous, well-defined and extended ordering of nanoscale structures. Indeed, the unique combination of adaptability towards other molecules and phases associated to the strong hydrogen-bond driven structure makes ionic liquids potential key tools in the preparation of a new generation of chemical nanostructures19,20 such as template porous silica prepared in a sol–gel process.21,22 Moreover, metal nanoparticles (MNPs) with small diameter and narrow size distribution can be prepared by simple H2 reduction of metal compounds or decomposition of organometallic species dissolved in ILs.23,24 In several cases the MNPs are not stable and tend to aggregate.25 Alternatively, these nanoparticles can be used in conjunction with other stabilizers or be easily transferred to other organic and inorganic supports to generate more stable and active catalysts.26–29 The nanoparticles/ionic liquid/stabilizer combination usually exhibits an excellent synergistic effect that enhances activity of the catalyst. So could be prepared more efficient and stable catalytic systems using the generation of metal nanoparticles associated with silica using ILs as templates for both catalytic partners i.e. the metal nanoparticles and the silica support.30–34
We present herein our results which show that imidazolium ionic liquids and functionalized ionic liquids, can be applied for the generation of the heterogeneous catalyst (Pt(0)/SiO2) via sol–gel processes. The heterogeneous catalyst obtained (Pt(0)/SiO2) was studied in hydrogenation reactions.
2. Experimental
2.1. General
All experiments were performed in air, except for the synthesis of the Pt(0) nanoparticles. The Pt(0) nanoparticles25,35 and the halide-free BMI·PF6,36a BMI·BF4 (ref. 36a) and PMI·Si·(OMe)3·X (X= Cl, N(Tf)2, PF6)36b ionic liquids were prepared according to literature procedure. Solvents, alkenes, and arenes were dried with the appropriate drying agents and distilled under argon prior to use. All other chemicals were purchased from commercial sources and used without further purification. Gas chromatography analysis was performed with a Hewlett–Packard-5890 gas chromatograph with an FID detector and a 30 m capillary column with a dimethylpolysiloxane stationary phase. The nanoparticles formation and hydrogenation reactions were carried out in a modified Fischer–Porter bottle immersed in a silicone oil bath and connected to a hydrogen tank. The temperature was maintained at 75 °C by a hot-stirring plate.2.2. Synthesis of Pt(0) nanoparticles supported in silica
Silica immobilized Pt(0) nanoparticles were prepared by the sol–gel method under acidic and basic conditions. Procedure for acid catalysis: 10 mL of tetraethoxy orthosilicate (9.34 g, 45 mmol) was introduced in a Becker under vigorous stirring at 60 °C. The Pt(0) nanoparticles (10 mg, 0.05 mmol) were dissolved in BMI·BF4 (1 mL, 5.1 mmol) and ethanol (5 mL). This solution was submitted to stirring and sonication for 2 min and then added to the solution containing TEOS. Consecutively, an acid solution (HF) was added as acid catalyst. The temperature was kept at 60 °C for 18 h. The resulting material was washed several times with acetone and dried under vacuum.Procedure for base catalysis: 10 mL of TEOS (9.34 g, 45 mmol) was added to ethanol (5 mL), containing the ionic liquid BMI·BF4 (1 mL, 5.1 mmol) and previously isolated Pt(0) nanoparticles (10 mg, 0.05 mmol). Then ethanol (95 mL) and ammonium hydroxide (20 mL) were added. The mixture was kept under stirring for 3 h at room temperature and left to stand for a further 18 h. The resulting xerogel was filtered and washed with acetone and dried under vacuum for 1 h.
2.3. X-ray diffraction (XRD)
The phase structures were characterized by of XRD Pt(0) nanoparticles. For XRD analysis, the nanoparticles were isolated as a fine powder and placed on the specimen holder. The XRD experiments were performed in a SIEMENS D500 diffractometer equipped with a curved graphite crystal using radiation Cu Kα (λ = 1.5406 Å). The diffraction data were collected at room temperature in Bragg–Brentano geometry θ–2θ. The equipment was operated at 40 kV and 20 mA with a scan range between 20° and 90°. The diffractograms were obtained with a constant step Δ2θ = 0.05. The indexation of Bragg reflections was obtained by fitting a pseudo-Voigt profile using the code FULPROFF code.37 Nanoparticles Pt(0)/SiO2 were analyzed on a glass substrate.2.4. Elemental analysis (CHN)
The organic phases present in the xerogels were analyzed using CHN elemental Perkin Elmer elemental CHNS/O analyzer, model 400. Triplicate analysis of the samples, previously heated at 100 °C under vacuum for 1 h, was carried out.2.5. Rutherford backscattering spectrometry (RBS)
Platinum loadings in catalysts were determined by RBS using He+ beams of 2.0 MeV incidents on homogeneous tablets of the compressed (12 MPa) catalyst powder. The method38 is based on the determination of the number and energy of the detected particles which are elastically scattered in the Coulombic field of the atomic nuclei in the target. In this study, the Pt/Si atomic ratio was determined by the heights of the signals corresponding to each of the elements in the spectra and converted to wt% Pt(0)/SiO2.2.6. Nitrogen adsorption–desorption isotherms
The adsorption–desorption isotherms of previous degassed solids (150 °C) were determined at liquid nitrogen boiling point in a volumetric apparatus, using nitrogen as probe. The specific surface areas of xerogels were determined from the t-plot analysis and pore size distribution was obtained using the BJH method. Homemade equipment with a vacuum line system employing a turbo-molecular Edwards's vacuum pump was used. The pressure measurements were made using a capillary Hg barometer and a Pirani gauge.2.7. Scanning electron microscopy (SEM) and electron dispersive spectroscopy (EDS) elemental analysis
The materials were analyzed by SEM using a JEOL model JSM 5800 with 20 kV and 5000 magnification. The same instrument was used for the EDS with a Noran detector (20 kV and acquisition time of 100 s and 5000 magnification).2.8. Transmission electron microscopy (TEM) analysis
The morphologies and the electron diffraction (ED) patterns of the obtained particles were determined on a JEOL JEM-2010 equipped with an EDS system and a JEOL JEM-120 EXII electron microscope, operating at accelerating voltages of 200 and 120 kV, respectively. The TEM samples were prepared by deposition of the Pt(0) nanoparticles or Pt(0)/SiO2 isopropanol dispersions on a carbon-coated copper grid at room temperature. The histograms of the nanoparticle size distributions were obtained from the measurement of around 300 diameters and reproduced in different regions of the Cu grid assuming spherical shapes.2.9. Catalytic hydrogenations
The catalysts (150 mg) were placed in a Fischer–Porter bottle and the alkene or arene (12.5 mmol) was added. The reactor was placed in an oil bath at 75 °C and hydrogen was admitted to the system at constant pressure (4 atm) under stirring until the consumption of hydrogen stopped. The organic products were recovered by decantation and analyzed by GC. The re-use of the catalysts were performed by simple extraction of the organic phase (upper phase) followed by the addition of the arene or alkene. After eleven recyclings the solid was isolated, re-dispersed in isopropanol and placed in a cooper carbon grid.3. Results and discussion
The sol–gel process is considered as a soft chemical approach for the synthesis of metastable oxide materials, this process allows us to obtain solid products by creating an oxide network via progressive polycondensation reactions of molecular precursors in a liquid medium.33,39 Essentially two steps are involved: hydrolysis and condensation. Both reactions are affected by the nature of the catalyst. Therefore, in the present study, two main routes were evaluated: (i) using an acid catalyst (HF), or (ii) using a base catalyst (NH4OH). In both routes, the hydrolysis and condensation of tetraethoxy orthosilicate (TEOS) were performed in the presence of Pt(0) nanoparticles, which were prepared by hydrogen reduction (4 atm) of Pt2(dba)3 dissolved in the ionic liquids at 75 °C.19 These nanoparticles obtained presented 2.3 nm of diameter. Fig. 1 shows the XRD pattern of Pt(0) nanoparticles and Pt(0) nanoparticles encapsulated in silica matrix showing the diffraction planes of silica and Platinum (Pt(0)/SiO2). This material was obtained by sol–gel synthesis under acidic conditions using the liquid having BMI·PF6 amount of Pt(0) <0.2% compared to silica.Table 1 presents the elemental analysis of the resulting platinum nanoparticles supported in silica Pt(0)/SiO2. Is possible observe that carbon and nitrogen content was taken as a sign for the presence of ionic liquids once encapsulated within the silica network (compare entries 2, 4, 6, 8 with entries 3, 5, 7, 9). Based on the results shown in Table 1, higher ionic liquid contents were incorporated with the acid catalysts. It is worth noting that in the case of acidic conditions, hydrolysis is faster than condensation.
| Entry | Sample | C/mmol g−1 | H/mmol g−1 | N/mmol g−1 | Pt(0)a % |
|---|---|---|---|---|---|
| a Determined by RBS. | |||||
| 1 | Pt/SiO2/NH4OH | 3.9 | 0.6 | 1.7 | 0.19 |
| 2 | Pt/SiO2/HF | 5.7 | 1.6 | 1.5 | 0.2 |
| 3 | Pt/SiO2/NH4OH/BMI·PF6 | 4.4 | 1.4 | 1.0 | 0.18 |
| 4 | Pt/SiO2/HF/BMI·Cl | 12.4 | 2.9 | 5.1 | 0.17 |
| 5 | Pt/SiO2/NH4OH/BMI·Cl | 9.9 | 2.1 | 4.1 | 0.2 |
| 6 | Pt/SiO2/HF/MIPSi(OMe)3·Cl | 18.7 | 3.3 | 3.8 | 0.18 |
| 7 | Pt/SiO2/NH4OH/MIPSi(OMe)3·Cl | 10.2 | 2.5 | 2.8 | 0.18 |
| 8 | Pt/SiO2/HF/MIPSi(OMe)3·N(Tf2) | 16.4 | 3.5 | 6.6 | 0.2 |
| 9 | Pt/SiO2/NH4OH/MIPSi(OMe)3·(NTf2) | 12.4 | 1.7 | 3.2 | 0.22 |
The rate of condensation slows down with increasing number of siloxane linkages around a central silicon atom. This leads to formation weakly branched polymeric networks. The condensation, in case of basic conditions, is more accelerated relative to hydrolysis. The rate of condensation increases with increasing number of siloxane bridges, resulting in the formation of highly branched networks.34,40 In the present case, based on the carbon and nitrogen contents, it seems that the resulting weakly branched structure generated in the presence of acid catalyst (either HF) guarantees the retention of the ionic liquid.
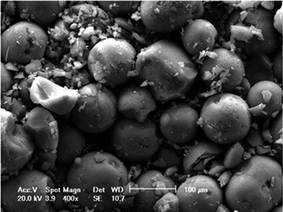
Rutherford backscattering spectrometry (RBS) was used in the determination of the metal contents. The Table 1 shows that the immobilized Pt content is roughly the same for silica prepared by both routes, corresponding to ca. 65–75% of the initial Pt content employed in the synthesis. The metal distribution in the support was determined by SEM-EDX analysis. The mapping showed a homogeneous Pt distribution in the silica grains, independently of the preparative route. The Fig. 2 shows SEM micrograph of Pt(0)/SiO2 synthesized using acid conditions by sol–gel method. The micrograph shows lighter regions, indicating the presence of platinum metal nanoparticles on the silica matrix (gray regions). The elemental composition of the region focused on the micrograph confirms this structure. Samples Pt(0)/SiO2 were analyzed by the scanning point and area exposed to the electron beam. All selected areas showed the presence Pt(0) in the silica matrix. In the micrograph, the metal is identified by the bright regions in contrast to the array of silicon that has the dark background. Fig. 3 illustrates the micrography of Pt(0)/SiO2 prepared by both routes, acid and basic. According to Fig. 3, particle morphologies are in accordance to that usually observed for pure silica synthesized by these routes. In the case of acid-catalyzed conditions, a less organized, plate-like structure was observed, while in the case of basic conditions, spherical particles were obtained (indeed shown in more detail in Fig. 4). It is worth noting that smaller particles were produced in the latter case.
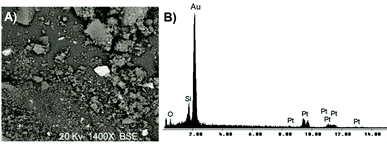 | ||
| Fig. 2 Micrograph obtained by SEM of the resulting xerogel: (A) Pt(0)/SiO2/HF, and EDX corresponding (B). | ||
 | ||
| Fig. 3 Micrographs obtained by M of the resulting xerogels: (A) Pt(0)/SiO2/HF (acid) and (B) Pt(0)/SiO2/NH4OH (basic). | ||
Transmission electron microscopy (TEM) was also employed for the characterization of the supported catalyst. Fig. 5 (top) shows the micrograph of the isolated Pt(0) particles, and their mean size, which was shown to be ca. 2.3 nm. In the case of Pt(0)/SiO2 Fig. 5 (bottom) prepared by acid catalysis (HF), both the morphology and size (ca. 2.3 nm) were maintained within the silica framework. It is very likely that the presence of ionic liquid affords stability, avoiding sintering of the metallic particles. The same behavior was observed for the material prepared by the base catalysis.
 | ||
| Fig. 5 TEM micrographs and histograms showing the particle size distribution of: (A) Pt(0) nanoparticles and (B) Pt(0)/SiO2/HF. | ||
The textural properties were further characterized by nitrogen adsorption. The specific area was calculated by the BET method, while pore diameter, by the BJH one (Table 2). According to Table 2, the silicas prepared in the absence of Pt(0) presented higher specific area (ca. 100 m2 g−1). The introduction of nanoparticles during the synthesis, independently of the synthetic route, led to a reduction in the specific area. The pore diameter was demonstrated to be smaller for the materials when NH4OH was used as catalyst. The pore volume was shown to be independent of the presence of Pt(0) in acidic or basic conditions. The supported catalysts were evaluated in hydrogenation reactions.
| E. | Sample | SBET/m2 g−1 | Vp/cm3 g−1 | dp/nm |
|---|---|---|---|---|
| a SBET = specific area determined by BET method. Vp = pore volume, dp = pore diameter. | ||||
| 1 | Pt(0)/SiO2/HF | 162 | 0.1 | 1.8 |
| 2 | Pt(0)/SiO2/HF/BMI·BF4 | 118 | 0.2 | 8.8 |
| 3 | Pt(0)/SiO2/HF/BMI·PF6 | 32 | 0.02 | 1.9 |
| 4 | Pt(0)/SiO2/NH4OH/BMI·BF4 | 106 | 0.2 | 4.8 |
| 5 | Pt(0)/SiO2/NH4OH | 102 | 0.07 | 4.3 |
| 6 | Pt(0)/SiO2/NH4OH/BMI·PF6 | 6.2 | 0.009 | 18 |
| 7 | Pt(0)/SiO2/HF/MIPSi(OMe)3·Cl | 66 | 0.03 | 3.9 |
| 8 | Pt(0)/SiO2/NH4OH/MIPSi(OMe)3·Cl | 193 | 0.2 | 2.8 |
| 9 | Pt(0)/SiO2/HF/MIPSi(OMe)3·N(Tf)2 | 98 | 0.3 | 5.2 |
| 10 | Pt(0)/SiO2/NH4OH/MIPSi(OMe)3·N(Tf)2 | 248 | 0.28 | 2.8 |
| 11 | Pt(0)/SiO2/HF/MIPSi(OMe)3·PF6 | 202 | 0.23 | 2.7 |
| 12 | Pt(0)/SiO2/NH4OH/MIPSi(OMe)3·PF6 | 239 | 0.27 | 2.8 |
Table 3 presents data regarding 1-decene, cyclohexene and benzene hydrogenation reactions. For comparative purposes we also included the data concerning the catalytic activity of isolated Pt(0) nanoparticles.19 In the benzene hydrogenations experiments, the product obtained was always the cyclohexane.
| Ent. | Catalyst | Ionic liquid | Substrate | Time (h) | TOF (h−1) | TOFc (h−1) |
|---|---|---|---|---|---|---|
| a Conditions: H2 pressure (4 atm), temperature 75 °C, ratio [alkene/arene]/[Pt(0)/SiO2] = 625/1, added Pt/SiO2 (150 mg, 0.025 mmol) Pt(0) followed by 12.5 mmol of alkenes or arenes used.b Pt(0) nanoparticles 10 mg, ratio [arene]/[Pt(0)] = 250/1, added Pt(0) (10 mg), followed by 12.5 mmol of the alkenes or arenes used. TOF values were calculated for 10% conversion.c TOF values corrected using the number of exposed surface metal atoms (39%). | ||||||
| 1 | aPt(0)/SiO2/HF | BMI·BF4 | 1-Decene | 0.4 | 156 | 400 |
| 2 | aPt(0)/SiO2/HF | BMI·BF4 | Cyclohexene | 0.8 | 78 | 200 |
| 3 | aPt(0)/SiO2/HF | BMI·BF4 | Benzene | 1.8 | 35 | 90 |
| 4 | aPt(0)/SiO2/NH4OH | BMI·BF4 | 1-Decene | 2.5 | 25 | 64 |
| 5 | aPt(0)/SiO2/NH4OH | BMI·BF4 | Cyclohexene | 6 | 11 | 28 |
| 6 | aPt(0)/SiO2/NH4OH | BMI·BF4 | Benzene | — | — | — |
| 7 | aPt(0)/SiO2/HF | MIPSi(OMe)3·N(Tf)2 | 1-Decene | 0.15 | 416 | 1067 |
| 8 | aPt(0)/SiO2/NH4OH | MIPSi(OMe)3·N(Tf)2 | Cyclohexene | 1.2 | 52 | 133 |
| 9 | aPt(0)/SiO2/HF | MIPSi(OMe)3·PF6 | 1-Decene | 0.6 | 104 | 267 |
| 10 | aPt(0)/SiO2/NH4OH | MIPSi(OMe)3·PF6 | 1-Decene | 0.8 | 78 | 200 |
| 11 | aPt(0)/SiO2/HF | MIPSi(OMe)3·Cl | 1-Decene | 0.5 | 125 | 320 |
| 12 | aPt(0)/SiO2/NH4OH | MIPSi(OMe)3·Cl | 1-Decene | 1.1 | 57 | 146 |
| 13 | aPt(0)/SiO2/HF | MIPSi(OMe)3·PF6 | 1-Decene | 0.7 | 90 | 230 |
| 14 | bPt(0) | — | 1-Decene | 0.9 | 28 | 72 |
| 15 | bPt(0) | — | Cyclohexene | 1.6 | 16 | 41 |
| 16 | bPt(0) | — | Benzene | 10 | 2.5 | 6.4 |
The catalytic activity of the heterogeneous catalysts studied in this work were expressed using the turnover frequency (TOF), (the TOF values were estimated for low substrate conversions, 10%), and that they should also be corrected by the number of exposed surface atoms by using the metal atom's magic number approach.41
Table 3 shows the results obtained in the hydrogenation reactions using the system Pt(0)/SiO2. For comparison effects were added results using only the Pt(0) nanoparticles in hydrogenation reactions (entry 21–23, Table 3). Is possible observe that all the supported systems were more active than those constituted of only Pt(0) nanoparticles. Among the silica-based systems, those prepared under acidic conditions are the most active, exhibiting higher TOF in comparison to those of isolated Pt(0) nanoparticles. The denser and bulkier structure generated under basic conditions might have afforded less active systems as shown by some clues. First, the ionic liquid content, which seems to be important in order to guarantee stability for the nanoparticles, was lower for these systems. Besides, according to porosimetric measurements, the pore diameter was much smaller for the Pt(0)/NH4OH/SiO2 system. Pt(0) encapsulated particles, in spite of a slightly higher content in comparison to that afforded with an acid catalyst (see Table 3), might be not accessible in the supported systems prepared under basic conditions. The hydrogenation of simple arenes and alkenes by Pt(0)/HF/SiO2 depends on steric hindrance at the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond and follows the same trend as observed with classical platinum complexes in homogeneous conditions, that is, the reactivity follows the order: terminal–internal. Finally, the catalytic material can be recovered by simple decantation and re-used at least eleven times without any significant loss in catalytic activity (see Fig. 6). The recovered material shows no sign of agglomeration as observed from the TEM analysis of Pt(0)/HF/SiO2 (representative image of the all sample observed by TEM) catalyst isolated after eleven recycles (Fig. 7).
C double bond and follows the same trend as observed with classical platinum complexes in homogeneous conditions, that is, the reactivity follows the order: terminal–internal. Finally, the catalytic material can be recovered by simple decantation and re-used at least eleven times without any significant loss in catalytic activity (see Fig. 6). The recovered material shows no sign of agglomeration as observed from the TEM analysis of Pt(0)/HF/SiO2 (representative image of the all sample observed by TEM) catalyst isolated after eleven recycles (Fig. 7).
 | ||
| Fig. 6 Conversion curves of 1-decene hydrogenation by Pt(0)/SiO2 nanoparticles at 4 atm H2 and 75 °C. | ||
 | ||
| Fig. 7 TEM micrographs of: (A) Pt(0)/HF/SiO2 isolated after eleven recycles and (B) histogram showing the particle size distribution. | ||
In the Fig. 6 is possible observe that loss of catalytic activity (observe recycles 6–8, Fig. 6) and after in nine recycle activity increases again. This fact can be related to the leaching of the surface of Pt(0) nanoparticles, cleaning the metal surface, making it active again.
4. Conclusions
The Pt(0) nanoparticles dispersed in ionic liquids and functionalized ionic liquids can be easily supported within a silica network using the sol–gel method (acid or base catalysis). The Pt(0) content in the resulting xerogels was shown to be independent of the preparative route, but acidic conditions afforded higher encapsulated ionic liquid content and xerogels with larger pore diameter, which in turn might have guaranteed higher catalytic activity in the hydrogenation of arenes and alkenes. The use of ionic liquid for the preparation of both nanoparticles and silica affords encapsulated ionic liquid/Pt(0)/SiO2 materials with different morphology, texture, and catalytic activity. A high level of ionic liquid incorporation seems to be important in order to guarantee stability for the nanoparticles. This combination exhibits an excellent synergistic effect that enhances the stability and activity of the Pt(0) hydrogenation catalysts. All the supported systems were more active than that constituted of isolated Pt(0) nanoparticles for the hydrogenation of arenes and alkenes.Acknowledgements
Thanks are due to the following Brazilian Agencies: CNPq, CAPES, FAPERGS for fellowships and partial financial support.Notes and references
- (a) D. Radivojevic, K. Seshan and L. Lefferts, Appl. Catal., A, 2006, 301, 51 CrossRef CAS PubMed; (b) V. D. Dao, C. Q. Tran, S. H. Ko and H. S. Choi, J. Mater. Chem. A, 2013, 1, 4436 RSC.
- (a) M. P. Kannan, T. Maiyalagan, N. G. Sahoo and M. Opallo, J. Mater. Chem. B, 2013, 1, 4655 RSC; (b) R. L. Moss, Platinum Met. Rev., 1967, 11, 141 CAS.
- (a) H. Hosseini, M. Mahyari and A. Bagheri, et al., J. Power Sources, 2014, 247, 70 CrossRef CAS PubMed; (b) S. Tanaka, N. Nagata, N. Tagawa and H. Hirata, et al., Dalton Trans., 2013, 42, 12662 RSC.
- C.-C. Kung, P.-Y. Lin and F. J. Buse, et al., Biosens. Bioelectron., 2014, 52, 1 CAS.
- Y. Wei, Z. Zhao and T. Li, et al., Appl. Catal., B, 2014, 146, 57 CrossRef CAS PubMed.
- (a) P. S. Roy and S. K. Bhattacharya, Catal. Sci. Technol., 2013, 3, 1314 RSC; (b) M.-Y. Kim, J.-H. Park, C.-H. Shin, S.-W. Han and G. Seo, Catal. Lett., 2009, 133, 288 CrossRef CAS.
- (a) P. Raybaud, C. Chizallet, H. Toulhoat and P. Sautet, Phys. Chem. Chem. Phys., 2012, 14, 16773 RSC; (b) A. Goguet, M. Aouine, F. J. Cadete, S. Aires, A. De Mallmann, D. Schweich and J. P. Candy, J. Catal., 2002, 209, 135 CrossRef CAS.
- C. Jiang, K. Hara and A. Fukuoka, Angew. Chem., Int. Ed., 2013, 52, 6265 CrossRef CAS PubMed.
- M. Nagai and R. D. Gonzalez, Ind. Eng. Chem. Prod. Res. Dev., 1985, 24, 525 CrossRef CAS.
- N. An, W. Zhang, X. Yuan, B. Pan, G. Liu, M. Jia, W. Yan and W. Zhang, Chem. Eng. J., 2013, 215, 1 CrossRef PubMed.
- A. Lemus, Y. V. Gómez and L. A. Contreras, Int. J. Electrochem. Sci., 2011, 6, 4176 Search PubMed.
- Y. Yang, J. Pan, N. Zheng, X. Liu and J. Zhang, Appl. Catal., 1990, 61, 75 CrossRef CAS.
- J. T. Miller, M. Schreier, A. J. Kropf and J. R. Regalbuto, J. Catal., 2004, 225, 203 CrossRef CAS PubMed.
- (a) T. Lopez, A. Romero and R. Gomez, J. Non-Cryst. Solids, 1991, 127, 105 CrossRef CAS; (b) C. P. Mehnert, Chem. –Eur. J., 2004, 11, 50 CrossRef CAS PubMed.
- M. A. Gelesky, S. S. X. Chiaro, F. A. Pavan, J. H. Z. Santos and J. Dupont, Dalton Trans., 2007, 5549 RSC.
- A. Riisager, R. Fehrmann, M. Haumann and P. Wasserscheid, Top. Catal., 2006, 40, 91 CrossRef CAS PubMed.
- J. Dupont and P. A. Z. Suarez, Phys. Chem. Chem. Phys., 2006, 8, 2441 RSC.
- C. S. Consorti, P. A. Z. Suarez, R. F. de Souza, R. A. Burrow, D. H. Farrar, A. J. Lough, W. Loh, L. H. M. da Silva and J. Dupont, J. Phys. Chem. B, 2005, 109, 4341 CAS.
- J. Dupont, J. Braz. Chem. Soc., 2004, 15, 341 CrossRef CAS PubMed.
- (a) R. Linhardt, Q. M. Kainz, R. N. Grass, W. J. Stark and O. Reiser, RSC Adv., 2014, 4, 8541 RSC; (b) M. Antonietti, D. B. Kuang, B. Smarsly and Z. Yong, Angew. Chem., Int. Ed., 2004, 43, 4988 CAS.
- Y. Zhou and M. Antonietti, J. Am. Chem. Soc., 2003, 125, 14960 CrossRef CAS PubMed.
- Y. Zhou, J. H. Schattka and M. Antonietti, Nano Lett., 2004, 4, 477 CrossRef CAS.
- Y. Zhou, Curr. Nanosci., 2005, 1, 35 CrossRef CAS.
- S. Dai, Y. H. Ju, H. J. Gao, J. S. Lin, S. J. Pennycook and C. E. Barnes, Chem. Commun., 2000, 3, 243 RSC.
- (a) C. W. Scheeren, G. Machado, J. Dupont, P. F. P. Fichtner and S. Texeira, Inorg. Chem., 2003, 42, 4738 CrossRef CAS PubMed; (b) C. W. Scheeren, G. Machado, S. R. Teixeira, J. Morais, J. B. Domingos and J. Dupont, J. Phys. Chem. B, 2006, 110, 13011 CrossRef CAS PubMed.
- E. T. Silveira, A. P. Umpierre, L. M. Rossi, G. Machado, J. Morais, G. V. Soares, I. L. R. Baumvol, S. R. Teixeira, P. F. P. Fichtner and J. Dupont, Chem. –Eur. J., 2004, 10, 3734 CrossRef CAS PubMed.
- J. Dupont, G. S. Fonseca, A. P. Umpierre, P. F. P. Fichtner and S. R. Teixeira, J. Am. Chem. Soc., 2002, 124, 4228 CrossRef CAS PubMed.
- J. Dupont and P. Migowski, Chem. –Eur. J., 2007, 13, 32 CrossRef PubMed.
- X. D. Mu, D. G. Evans and Y. A. Kou, Catal. Lett., 2004, 97, 151 CrossRef CAS.
- X. D. Mu, J. Q. Meng, Z. C. Li and Y. Kou, J. Am. Chem. Soc., 2005, 127, 9694 CrossRef CAS PubMed.
- S. D. Miao, Z. M. Liu, B. X. Han, J. Huang, Z. Y. Sun, J. L. Zhang and T. Jiang, Angew. Chem., Int. Ed., 2006, 45, 266 CrossRef CAS PubMed.
- J. Huang, T. Jiang, B. X. Han, W. Z. Wu, Z. M. Liu, Z. L. Xie and J. L. Zhang, Catal. Lett., 2005, 103, 59 CrossRef CAS.
- V. Mevellec, A. Nowicki, A. Roucoux, C. Dujardin, P. Granger, E. Payen and K. Philippot, New J. Chem., 2006, 30, 1214 RSC.
- K. Zhu, F. Pozgan, L. D'Souza and R. M. Richards, Microporous Mesoporous Mater., 2006, 91, 40 CrossRef CAS PubMed.
- K. Moseley and P. M. Maitlis, J. Chem. Soc. Chem. Commun., 1971, 982 RSC.
- (a) C. C. Cassol, G. Ebeling, B. Ferrera and J. Dupont, Adv. Synth. Catal., 2006, 348, 243 CrossRef CAS PubMed; (b) Y. Kume, K. Qiao, D. Tomida and C. Yokoyama, Catal. Commun., 2008, 9, 369 CrossRef CAS PubMed.
- J. R. Carbajal, Short Reference Guide of The Program Fullprof version 3.5, 2003, http://Charybde.Saclay.Cea.Fr Search PubMed.
- F. C. Stedile and J. H. Z. dos Santos, Phys. Status Solidi A, 1999, 173, 123 CrossRef CAS.
- U. Schubert and N. Husing, Inorganic Materials: A Chemical Approach, Wiley, Weinheim, 1st edn, 2000 Search PubMed.
- Characterization and Chemical Modification of the Silica Surface. Studies in Surface Science and Catalysis, ed. E. F. Vansant, P. Van Der Voort and K. C. Vrancken, Elsevier, Amsterdam, 1995, vol. 93 Search PubMed.
- A. P. Umpierre, E. Jesús and J. Dupont, ChemCatChem, 2011, 3, 1413 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |