Design and controlled synthesis by dual polymerization of new organic–inorganic hybrid material for photonic devices
Saly Yaacoub*ab,
Sylvie Calas-Etiennea,
Jihane Jabbourb,
Kassem Amroa,
Rabih Taukb,
Antonio Khouryb,
Ahmad Mehdic and
Pascal Etiennea
aCharles Coulomb Laboratory, University of Montpellier 2, Place E. Bataillon, UMR 5221, cc074, 34095 Montpellier Cedex 5, France. E-mail: saly.yaacoub@univ-montp2.fr
bPlatform for Research in Nanosciences and Nanotechnology, Lebanese University, Campus Pierre Gemayel, Fanar-Metn, BP90239-Lebanon
cInstitute Charles Gerhardt, Chimie Moléculaire et Organisation du solide, UMR 5253, cc1701, Place Eugène Bataillon, 34095 Montpellier Cedex 5, France
First published on 28th March 2014
Abstract
Organic–inorganic hybrid material was synthesized by double polymerization processes i.e. a sol–gel process and organic polymerization respectively. For this study, hybrid monomer, 4-vinyl ether-phenyltriethoxysilane (VEPTES) was used as starting building block. First, the silica matrix with tunable ratio of siloxane and silanol units was synthesized by a sol–gel process under acidic conditions and the organic network was formed by cationic photopolymerization of vinyl ether groups. Mineral and organic polymerization kinetics were respectively monitored by liquid 29Si-NMR and IR spectroscopy. The effect of the silicate backbone on the organic photopolymerization process was studied and elucidated. The optical performance of this new hybrid material has been studied using the near-infrared spectroscopy.
Introduction
Organic–inorganic hybrid materials are of central attention in many fields since they combine both characteristics of inorganic and organic materials.1–7 ORganically MOdified SILlicates (ORMOSILs) materials are generally obtained from a molecular precursor R′Si(OR)3 which contains two reactive parts and offers two polymerization (inorganic and organic) processes. The inorganic network is obtained by hydrolysis and polycondensation of trialkoxysilyl groups Si(OR)3 (sol–gel process) while the organic network is generated by the photopolymerization of the reactive organic function (vinyl, allyl, epoxy…) of the R′ part. In contrast to radical polymerization, cationic polymerization is not inhibited by oxygen, but can be affected by the presence of hydroxyl groups which act as nucleophiles.8Among cationically cured systems, vinyl ether is the most reactive monomer known in this type of polymerization.9 Using an appropriate initiator, cationic polymerization of vinyl ether is defined as an additional polymerization reaction mediated by propagation carbocation derived from a vinyl ether monomer initiated by strong Brönsted acid. Common cationic photoinitiators are based on diaryliodonium or triarylsulfonium salts.10–12 These strong protonic acids are generally obtained from the photolysis of diaryliodonium or triarylsulfonium salts bearing weakly coordinating anions such as hexafluoroarsenate anion AsF6− or hexafluorophosphate PF6−.
Organic–inorganic hybrid materials are particularly attractive for photonics and integrated optics13–15 but the main factor limiting the development of these devices is the propagation losses. In fact, in organic–inorganic hybrid materials synthesized by a sol–gel process,16 the presence of OH groups underlies the attenuation at the second and the third telecommunication windows. In addition, the presence of CH aliphatic groups, in the precursor, can contribute to the absorption at some wavelengths.17
The most powerful experimental technique for studying silicon-based sol–gel chemistry remains the nuclear magnetic resonance (29Si-NMR).3,18 An 29Si-NMR of a sol–gel mixture allows the identification and quantification of all species present in solution. Hydrolysis and condensation reactions of VEPTES-based sol have been studied as a function of several synthesis parameters (temperature, time, pH and concentration) using 29Si-NMR spectroscopy and reported recently.19 We have shown clearly the influence of these different parameters on the kinetic reactions in order to obtain a complete hydrolysis of all alkoxide units with the highest possible condensation rate.
Besides the mineral polymerization we investigate, in this paper, the photoinitiating polymerization behaviors of vinyl ether units according to a cationic mechanism. The quantitative study of the vinyl ether conversion was based on infrared spectra collected in the range (400–4000 cm−1).
Versace et al. have studied the relation between organic and inorganic network polymerizations.20 They reported that an increase of inorganic condensation degree induces a decrease of organic conversion rate. For these reasons, synthesis conditions have to be carefully chosen to ensure both a sufficient degree of mineral condensation and an important organic conversion rate.
This paper deals with the structural characterization of double polymerization process for the two reactive parts of a new hybrid precursor. In order to reduce the amount of groups involved in the attenuation, the new synthesized precursor contains a reduced number of aliphatic CH groups in comparison with other hybrid precursors.19,21 In parallel, mineral polymerization has been followed through 29Si-NMR spectroscopy in order to reduce the presence of OH groups. In this respect, near infrared spectroscopy was used to study the performance of this new hybrid material at the optical transmission windows.
Experimental section
Materials
All reagents were purchased from Alfa Aesar. Tetrahydrofuran was distilled from sodium benzophenone immediately before use. The 1H, 13C and 29Si-NMR spectra were recorded on a Bruker Advance 200 DPX spectrometer. The bifunctional precursor 4-vinyletherphenyltriethoxysilane (VEPTES) was used as starting monomer. It was prepared by silylation of 4-bromophenylvinylether according to Scheme 1.Synthesis of 4-bromophenylvinylether
In a 1 L two neck flask, (318 g, 1.7 mol) of dibromoethane and (109 g, 0.80 mol) of potassium carbonate were mixed in 500 mL of acetonitrile. A solution of 4-bromophenol (67 g, 0.38 mol) in acetonitrile (70 mL) was slowly dropped in the mixture at 0 °C (ice bath) which was then heated under reflux for one night. Once the solution was cooled down to room temperature, filtered and concentrated, an orange solid substance was obtained. The solid was crystallized from methanol to give 50 g (0.18 mol) of 1-bromo-4-(2-bromoethoxy) benzene. This last one was dissolved in 70 mL of dried tetrahydrofuran (THF) and a solution of potassium tertbutoxide (27 g, 0.24 mol) in THF (80 mL) at 0 °C under argon atmosphere. After stirring at room temperature for one night, the mixture was filtered and the obtained residue was dissolved in pentane and extracted with water (3 × 100 mL). The combined extracts were dried over MgSO4, and concentrated under vacuum to give 4-bromophenylvinylether 2, a slightly yellow oil. Yield: 76%. 1H NMR (200 MHz, CDCl3) δ ppm: 7.46 (d, J = 8 Hz, 2H, Ar), 6.92 (d, J = 8 Hz, 2H, Ar), 6.63 (dd, 1H, HC = ), 4.83 (d, J = 8 Hz, 1H, H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 4.52 (d, J = 8 Hz, 1H, H2C
), 4.52 (d, J = 8 Hz, 1H, H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ); 13C NMR (50 MHz, CDCl3) δ ppm: 155.84 (Ar), 147.72 (Ar), 132.57 (Ar), 118.84 (Ar), 115.63 (HC
); 13C NMR (50 MHz, CDCl3) δ ppm: 155.84 (Ar), 147.72 (Ar), 132.57 (Ar), 118.84 (Ar), 115.63 (HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 95.93 (H2C
), 95.93 (H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ).
).
Synthesis of 4-vinyletherphenyltriethoxysilane (VEPTES)
A solution of 4-bromophenylvinylether 2 (55.0 g, 0.276 mol) in THF (115 mL) was dropped at room temperature into a three neck flask containing (8.6 g, 0.360 mol) of magnesium and 70 mL of dried THF. The resulting mixture was heated under reflux for 2 hours. After being cooled to room temperature, the ashen solution was dropped into a 1 L two neck flask containing a solution of tetraethoxysilane (229.0 g, 1.10 mol) in THF (300 mL) and cooled at −30 °C. After 48 hours of stirring at room temperature, the mixture was filtrated and the filtrate was concentrated under vacuum to give yellow oily curd crude. After distillation under vacuum, 4-vinyletherphenyltriethoxysilane (VEPTES) was obtained as a colorless oil. Yield: 71%. Bp: 90–92 °C at 0.04 mmHg. 1H NMR (200 MHz, CDCl3) δ ppm: 7.70 (d, J = 8 Hz, 2H, Ar), 7.07 (d, J = 8, 2H, Ar), 6.72 (dd, 1H, HC = ), 4.87 (dd, 1H, H2C = ), 4.52 (dd, 1H, H2C = ), 3.92 (q, 6H, CH2O), 1.30 (t, 9H, CH3); 13C NMR (50 MHz, CDCl3) δ ppm: 158.62 (Ar), 147.48 (Ar), 136.57 (Ar), 124.97 (Ar), 116.34 (HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 95.88 (H2C
), 95.88 (H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 58.72 (CH2O), 18.12 (CH3); 29Si NMR (40 MHz, CDCl3) δ ppm: −57.3.
), 58.72 (CH2O), 18.12 (CH3); 29Si NMR (40 MHz, CDCl3) δ ppm: −57.3.
Igracure 250 (iodonium, (4-methylphenyl)[4-(2-methylpropyl)-phenyl]-hexafluorophosphate) and Darocure ITX (mixture of 2-isopropyltrioxanthone and 4-isopropylthioxanthone) were purchased from Ciba Speciality Chemicals Inc. The initiator concentrations were between 1 and 5 wt% with respect to the vinyl ether monomer mass. A thioxanthone photosensitizer was used to expand the spectral region over which the cationic initiator is not effective. Typically, photosensitizers make it possible to initiate the photopolymerization using near-UV or even visible wavelengths of light.22–25 Other than iodonium-based PAG, (tris[4-(4-acetylphenyl)sulfanylphenyl]sulfonium tris(trifluoromethylsulfonyl)methide), GSID26-1, is used in this study. These two photoactives compositions, Irgacure 250/Darocure ITX and PAG GSID26-1, are used to carry out the cationic photopolymerization of vinyl monomers with long-wavelength UV light at 365 nm. Molecular structures of the cationic photoinitiator and photosensitizer are shown in Fig. 1.
Sol synthesis
Based on previous works on phenyltriethoxysilane26 and in order to obtain a complete hydrolysis of the three alkoxide units with the highest possible condensation rate, it is important to work in an acidic medium with a high hydrolysis rate . Under these conditions, hydrolysis is fast compared to condensation reaction, most of alkoxy groups are converted to hydroxyl groups leading to the formation of a highly condensed silicate polymers.27–29
. Under these conditions, hydrolysis is fast compared to condensation reaction, most of alkoxy groups are converted to hydroxyl groups leading to the formation of a highly condensed silicate polymers.27–29
The starting sol corresponds to the precursor hydrolysis rate of 8 in aqueous HCl solution of 10−2 M (see Fig. 2). A ratio (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of (ethyl alcohol (99%)
1) of (ethyl alcohol (99%)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VEPTES) is necessary to obtain a homogeneous solution. For this, two solutions (VEPTES
VEPTES) is necessary to obtain a homogeneous solution. For this, two solutions (VEPTES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol: 1
ethanol: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) and (HCl
8) and (HCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol: 8
ethanol: 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) were prepared. The obtained HCl–ethanol solution was dropped under stirring to the VEPTES–ethanol.
8) were prepared. The obtained HCl–ethanol solution was dropped under stirring to the VEPTES–ethanol.
After the complete addition of the acidic solution, the mixture was continuously stirred for 1, 2 and 3 days at 60 °C. Afterwards, the solvent was removed by rotavapor at 30 °C under vacuum (42 mbar) to obtain a dry extract value about 55%. Finally, the appropriate photoinitiator and photosensitizer were added to the obtained sol.
Characterization
In the sol–gel reaction of the precursor, the ethoxy silyl groups are transformed into hydroxyl groups and siloxane bonds by hydrolysis and polycondensation, respectively. Classical Tji notation is used for the different trifunctional silicate species depending on the number of oxygen bridging atoms surrounding the central silicon atom. i represents the number of siloxanes and j the number of silanols.
IR analyses were performed to investigate the conversion rate of vinyl ether monomers under UV irradiation. Transmission FTIR spectroscopy is the mostly used method to monitor the photopolymerization process of UV-cured coatings. Thick films are shown to be unsuitable for structural investigation using transmission IR spectroscopy, saturation bands quickly appears for such films. To avoid this problem, the film thickness should be around 1 μm. FTIR spectra were recorded on a Nicolet FTIR-spectrometer 510p in the medium infrared range of 4000–400 cm−1. The sample was inserted into a slide frame and placed in the compartment of the spectrophotometer. The experiments were conducted at room temperature in absorption mode at 8 cm−1 resolution with 32 scans per spectrum to reduce the noise.
The evolution of vinyl ether conversion α with time, t, was calculated by the following equation:32
 | (1) |
| A = εlC | (2) |
Using eqn (1) and (2) the degree of curing of vinyl ether groups is determined at any time according to the following equation:
 | (3) |
The evolution of the vinyl ether absorbance is compared to a reference vibration band, independent from the curing process.
Results and discussion
Mineral polymerization
Liquid 29Si nuclear magnetic resonance spectroscopy was used to characterize the structural evolution of the mineral network during the sol–gel synthesis.19For the following spectra, chemical shifts for the species formed during these reactions are listed in Table 1.
Fig. 3 presents 29Si-NMR spectra of concentrated sols a, b and c.
We have already shown that working at 60 °C is the best way to enhance the sol–gel reaction.19 In each spectrum described in Fig. 3, the hydrolysis species disappear, leaving place to the condensation ones. T1, T2 and T3 species and their concentration are summarized in Table 2.
| Species | Aging time | |||||
|---|---|---|---|---|---|---|
| Sol a | Sol b | Sol c | ||||
| (%)′′ | (%)′′′ | (%)′′ | (%)′′′ | (%)′′ | (%)′′′ | |
| a ′′: % before concentration, ′′′: % after concentration. | ||||||
| T20 | 1 | — | 1 | — | 0.4 | — |
| T30 | 8 | — | 1 | — | 0.6 | — |
| T1 | 35 | 24 | 34 | 15 | 28 | 7 |
| T2 | 53 | 58 | 54 | 64 | 55 | 67 |
| T3 | 3 | 18 | 10 | 21 | 16 | 26 |
Condensation rate  (%) (%) |
50 | 65 | 57.3 | 69 | 62 | 73 |
The concentration of the sol after 1 day induces a decrease of 11% of T1 species for the benefit of T2 and T3 species which increase respectively by 5% and 15% respectively. The same evolution was observed for both sols b and c. It is worth noting that, the sol concentration induces a decrease of T1 species for the benefit of T2 and T3 species and subsequently an increase in the condensation rate of 12% and 11% respectively. This evolution highlights the effect of the sol concentration on the condensation of silanol groups (Si–OH) and the formation of siloxane units (Si–O–Si).
Besides the synthesis of mineral network, cationic photopolymerization process allows the building of organic network. In order to choose the optimized sol, the correlation between the formation of inorganic and organic phases was studied for the last three solutions.
Organic polymerization
This part is dedicated to the investigation of UV curing behaviors using the FTIR spectroscopy. The samples were prepared by spin-coating a VEPTES photoactive based solution on a silicon wafer.Fig. 4 shows the spectrum of the film before UV curing in middle infrared region. The characteristic absorption bands of interest are reported in Table 3.
| Wavenumber (cm−1) | Characteristics vibration |
|---|---|
| 1000–1130 | Si–O asymmetric stretching of Si–O–Si |
| 1645 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching of vinyl ether bond C stretching of vinyl ether bond |
| 1509/1595 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching of aromatic bond C stretching of aromatic bond |
| 3390 | OH stretching |
The conversion rate was calculated using eqn (3). In this case, the double bond conversion rate during the cationic photopolymerization of VEPTES was determined according to the characteristic absorption band of vinyl ether centered at 1645 cm−1 and to the aromatic double bond C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C at 1509 cm−1 used as reference for the normalization of vinyl ether peak absorbance. The Avinyl and Aref for t = 0 were determined from the spectrum recorded just after the deposition of the film on the silicon substrate.
C at 1509 cm−1 used as reference for the normalization of vinyl ether peak absorbance. The Avinyl and Aref for t = 0 were determined from the spectrum recorded just after the deposition of the film on the silicon substrate.
Influence of the aging time of the sol
Structural investigation of UV-cured films based on VEPTES begins by the characterization of vinyl ether-conversion rate versus the aging time of the starting sol. The photopolymerization process of vinyl ether groups has been studied using an UV LED33 lamp with an emission spectrum at 365 ± 5 nm and with an illumination equal to 25 mW cm−2 ± 10%.To make sol a, b and c sensitive at 365 nm, Irgacure 250/Darocure ITX was added by respective weight percentages 5% and 1% of VEPTES.
Fig. 5 shows the conversion rate versus exposure time for UV-cured films deposited using sols a, b and c.
 | ||
| Fig. 5 Vinyl ether conversion curves of sols a, b and c in function of irradiation time. Inset: conversion rate versus condensation rate. | ||
For all sols, the conversion rate increased with the exposure time. A similar behavior has been observed previously for vinyl ether-based photosensitive films.34
In addition, it was clearly shown that the organic and inorganic polymerizations are related (see inset Fig. 5). There was a significant slowing of the vinyl ether conversion rate with increasing sol aging. The vinyl ether conversion decreased from 72% to 29%, when the inorganic condensation degree increased from 65% to 73%.
The mobility of the polymerizable groups is reduced by the increase of the condensation rate of mineral network. In other words, the reactive species of organic network are fixed by the inorganic network which prevents their propagation to other active monomers. The similar effect has been already observed in the literature in the case of epoxy polysilsesquioxane resins.20,35
From these observations, sol a seems to be the most suitable sol to obtain the minimum residual silanol (Si–OH) groups and in the same time the highest possible condensation rate. The amount of OH groups is necessary to ensure a good adhesion of the coating adhesion on silicon substrate.
Influence of the photoactive composition
The conversion rate has been also studied as a function of the photoactive composition. For this investigation, two systems were tested for their efficiency to start the curing reaction of VEPTES-based resins at 365 nm. Fig. 6 presents the vinyl ether conversion curves versus irradiation time for VEPTES UV-cured films using two photopolymerizable compositions. For the first one, the photoinitiator PAG GSID26-1 was added to the concentrated sol a at a 5% wt of VEPTES. | ||
| Fig. 6 Vinyl ether conversion curves versus irradiation time for VEPTES UV-cured films with two photoactive compositions. | ||
For the second one, the couple Irgacure 250/Darocure ITX was added by respective weight percentages 5% and 1% of VEPTES.
It was observed for both compositions, that the conversion rate increased with the exposure time with better reactivity for the second composition (Irgacure 250/Darocure ITX). With the first composition, the conversion reached 26% after 60 s, while the couple Irgacure 250/Darocure ITX reached 70%. Then, it continues to grow and reached 81% after 145 s, instead of 40% for the first one.
It is well known, with UV irradiation, that diaryliodonium and triarylsulfonium salts undergo a photodissociation which leads to the generation of a strong Brönsted acid. The produced strong acid is responsible of the initiation of cationic polymerization by the direct protonation of the monomer. The strength of the acid depends on the character of the anion present on the starting onium salt. Maximum rates of polymerization are achieved when the anion is nonnucleophilic.36
Since, the strength of the acid is related to conjugate formed-base. Thereby, the low reactivity of GSID26-1 in comparison to that of Irgacure 250/Darocure ITX-based composition is related to the stronger nucleophilic character of (CF3SO2)3C− compared to that of PF6−.
Based on these observations, the couple Irgacure 250/Darocure ITX was chosen for the next study.
Influence of photolithography parameters
According to the standard process of soft lithography, after deposition, the photoresist coating is subjected to a soft-bake. The soft-bake SB is done to drive away the solvent from the liquid film in order to improve the adhesion on the silicon wafer.Fig. 7 presents the conversion rate of the photoresist coating as a function of exposure time for different baking times at 60 °C. Four photoresist films deposited on silicon substrate underwent a soft bake respectively for 0, 1, 3 and 5 min at 60 °C. After recording the spectrum at t = 0 as a reference, each of these layers is studied as a function of exposure time at 365 nm using IR spectroscopy in the middle range.
 | ||
| Fig. 7 Vinyl ether conversion curves versus irradiation time for VEPTES UV-cured films for different SB times at 60 °C. | ||
It was observed that the conversion rate increased with the exposure time for any SB annealing time (see Fig. 7). However, the evolution is not proportional. The conversion rate decreases with the increase of the annealing time SB. After 145 s, the conversion rate reached 81%, 75%, 64% and 61% for layers respectively after 0, 1, 3 and 5 minutes at 60 °C.
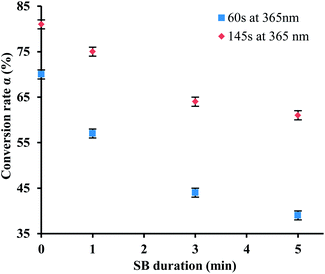
The relation between the conversion rate and the annealing time SB were summarized in Fig. 8 for two exposure time at 365 nm.
This figure proves the influence of the SB annealing time on the conversion rate and subsequently the influence of the crosslinking of mineral network. When the crosslinking of inorganic network is more advanced, the conversion of organic species is less important. This supports our previous observations.
Associated with a high conversion rate, the rate of polymerization reaction is a key parameter in photolithography process. Fig. 9 represents the variation of the polymerization rate with the SB annealing time. The rate of polymerization reaction is calculated from the slope of each curve in Fig. 7 between 0 s and 50 s.
This study shows that the rate of polymerization reaction decreases with the increase of SB annealing time. Hence, SB annealing time has a significant influence on the crosslinking of the inorganic network, which obviously affects the conversion rate of the vinyl ether function and also the rate of polymerization reaction. A high conversion rate must be obtained by a short SB annealing time.
Till now, we have studied the evolution of the conversion rate during the first stage of the photolithography process, mainly the Soft-Bake (SB). It is important to study the evolution throughout the process, from the exposure step and Post-Exposure Bake (PEB) until the densification treatment, Hard-Bake (HB).
The evolution of vinyl ether-conversion rate at every step of the photolithographic process is represented in Fig. 10. We chose to perform this study on a layer without the first annealing SB. The circle dots correspond to the exposure step, the square dots to the PEB step carried out at 80 °C and the triangle one for the HB stage performed at 100 °C.
Fig. 10 shows clearly the impact of each step in the photolithography process on the conversion rate of organic function. At first, α increases very rapidly with exposure time. It increases from 7% after 5 s to 72% after one minute of exposure. This evolution shows that an increase of the exposure time enhances greatly the polymerization of vinyl ether functions. This part of the curve is similar to that observed on Fig. 6 for the second photoactive composition Irgacure 250/Darocure ITX. Thus, the result shows the excellent reproducibility of the solution behavior.
After exposure for 60 seconds, we studied the effect of each annealing, PEB and HB. The conversion rate continues to increase up to be 92% after 5 min at 80 °C and 100% just after the first fifteen minutes at 100 °C (during the HB).
Performance of VEPTES-based coating at 1310 and 1550 nm
In order to use this material for integrated optical devices, optical performances were studied in the near infrared region. The near-infrared spectral range (800 to 2500 nm) contains the two optical transmission windows located at 1310 and 1550 nm allowing the absorption study of the material.The films were deposited on glass microscope slides, which have been previously treated with piranha solution.
Fig. 11 presents the spectrum obtained in the near infrared region for VEPTES-based coating.
This spectrum shows the presence of several absorption bands (overtones and combination bands of fundamental molecular vibrations), the main ones are shown in Table 4. The various absorption bands due to overtones and combinations bands of CH and OH groups.
| λ (nm) | Vibration mode |
|---|---|
| 1369 | OH of Si–OH (elongation) |
| 1380–1450 | 2ν CH (CH2 or CH3) + δ CH (CH2 or CH3) |
| 1615 | 2ν CH (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) CH2) |
| 1680 | 2ν CH (CH2 or CH3) |
| 1915 | OH of H2O exterior (deformation and elongation) |
The centered band at 1680 nm corresponds to the first harmonic (2ν) of CH bonds in CH2 and CH3 groups. The band at 1615 nm corresponds to the first harmonic (2ν CH) linked to the vinylic CH groups. The band between 1380 and 1450 nm is due to many possible combinations between the first harmonic (2ν CH) and the bending (δ CH) of CH aliphatic groups. Then, the presence of these groups contributes slightly to the absorptions at 1310 and 1550 nm (observed at the foot of the band). Therefore, VEPTES-based film has low level of absorptions at 1310 and 1550 nm.
Conclusions
In this work, the dual polymerization of VEPTES resins was investigated. The resulting mineral network was synthesized under acidic conditions via the sol–gel process, followed by an organic photopolymerization in presence of a diaryliodonium salt. Sol–gel reactions were monitored by liquid 29Si-NMR spectroscopy and the photocuring behavior of vinyl ether based monomers was investigated using infrared spectroscopy in the middle range.The correlation between mineral and organic polymerization has been studied to ensure both a sufficient degree of polymerization. A significant slowdown of vinyl ether conversion rates with increasing sol aging was observed.
Finally, the optical performance of VEPTES-based resin has been studied using the near-infrared spectroscopy. Results show a promising transmission level for the use of VEPTES-based film at the second and the third telecommunication windows. Optical losses measurements are under investigation.
Acknowledgements
The authors would like to acknowledge the financial support provided by the Lebanese National Council for Scientific Research CNRS (via the Grant Research program) and the social foundation Azm and Saade (via a Program of cooperation with the Lebanese university).Notes and references
- C. Sanchez, B. Julian, P. Belleville and M. Popall, J. Mater. Chem., 2005, 15, 3559 RSC
.
- C. Sanchez, P. Belleville, M. Popall and L. Nicole, Chem. Soc. Rev., 2011, 40, 696 RSC
.
- P. Judeinstein and C. Sanchez, J. Mater. Chem., 1996, 6, 511 RSC
.
- B. Arkles, MRS Bull., 2001, 26, 402 CrossRef CAS
.
- G. Schottner, Chem. Mater., 2001, 13, 3422 CrossRef CAS
.
- E. Besson, A. Mehdi, C. Reyé, R. Corriu and A. Gibaud, Silicon Based Polym., Springer Science, 2008, p. 223 Search PubMed
.
- A. Mehdi, C. Reye and R. Corriu, Chem. Soc. Rev., 2011, 40, 563 RSC
.
- A. D. Jenkins and A. Ledwith, Reactivity, mechanism and structure in polymer chemistry, John Wiley and Sons, London, 1974 Search PubMed
.
- C. Decker, C. Bianchi, D. Decker and F. Morel, Prog. Org. Coat., 2001, 42, 253 CrossRef CAS
.
- J. V. Crivello, J. Polym. Sci., Part A: Polym. Chem., 1999, 37, 4241 CrossRef CAS
.
- J. V. Crivello and J. H. W. Lam, Macromolecules, 1977, 10, 1307 CrossRef CAS
.
- Y. Yagci and I. Reetz, Prog. Polym. Sci., 1998, 23, 1485 CrossRef CAS
.
- H. Krug, F. Tiefensee, P. W. Oliveira and H. K. Schmidt, Sol–Gel Optics II, Proc. SPIE, 1992, vol. 1758, p. 448 Search PubMed
.
- C. Sanchez and P. Gomez-Romero, Functional hybrid materials, WILEY-VCH, Weinheim, 2004 Search PubMed
.
- H. Schmidt and M. Popall, Sol–gel optics, Proc. SPIE, 1990, vol. 1328, p. 249 Search PubMed
.
- M. Oubaha, P. Etienne, S. Calas, P. Coudray, J. M. Nedelec and Y. Moreau, J. Sol–Gel Sci. Technol., 2005, 33, 241 CrossRef CAS
.
- M. Oubaha, P. Etienne, P. Boutinaud, J. M. Nedelec, P. Coudray and Y. Moreau, Opt. Mater., 2006, 28, 502 CrossRef PubMed
.
- F. Babonneau and J. Maquet, Polyhedron, 2000, 19, 315 CrossRef CAS
.
- S. Yaacoub, S. Calas-Etienne, J. Jabbour, R. Tauk, A. Khoury, A. Mehdi and P. Etienne, J. Sol–Gel Sci. Technol., 2013, 67, 384 CrossRef CAS
.
- D. L. Versace, A. Chemtob, C. Croutxé-Barghorn and S. Rigolet, Macromol. Mater. Eng., 2010, 295, 355 CrossRef CAS PubMed
.
- J. Jabbour, S. Calas-Etienne, M. Smaihi, S. Gatti, R. Kribich, G. Pille, Y. Moreau and P. Etienne, Appl. Surf. Sci., 2007, 253, 8032 CrossRef CAS PubMed
.
- J. V. Crivello, Adv. Polym. Sci., 1984, 62, 1 CrossRef CAS
.
- W. D. Cook, S. Chen, F. Chen, M. U. Kahveci and Y. J. Yagci, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 5474 CrossRef CAS PubMed
.
- J. D. Cho and J. W. Hong, J. Appl. Polym. Sci., 2005, 97, 1345 CrossRef CAS PubMed
.
- J. V. Crivello and U. Bulut, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 5217 CrossRef CAS PubMed
.
- Y.-S. Li, Y. Wang and S. Ceesay, Spectrochim. Acta, Part A, 2009, 71, 1819 CrossRef PubMed
.
- C. J. Brinker, K. D. Keefer, D. W. Schaefer and C. S. Ashley, J. Non-Cryst. Solids, 1982, 48, 47 CrossRef CAS
.
- R. A. Assink and B. D. Kay, Annu. Rev. Mater. Sci., 1991, 21, 491 CrossRef CAS
.
- L. W. Kelts, N. J. Effinger and S. M. Melpolder, J. Non-Cryst. Solids, 1986, 83, 353 CrossRef CAS
.
- S. K. Mah and I. J. Chung, J. Non-Cryst. Solids, 1995, 183, 252 CrossRef CAS
.
- R. J. Hook, J. Non-Cryst. Solids, 1996, 195, 1 CrossRef CAS
.
- E. G. Karayannidou, D. S. Achilias and I. D. Sideridou, Eur. Polym. J., 2006, 42, 3311 CrossRef CAS PubMed
.
- http://www.kloe.fr/en/our-solutihttp://www.kloe.fr/fr/nos-solutions-lithographie/gamme-uv-kub, Gamme UV-KUB 2013.
- A. Chemtob, C. Belon, C. Croutxé-Barghorn, S. Rigolet, L. Vidal, J. Brendlé, J. Mandel and N. Blanchard, Polym. Eng. Sci., 2011, 51, 1466 CAS
.
- J. Chen and M. D. Soucek, J. Appl. Polym. Sci., 2003, 90, 2485 CrossRef CAS PubMed
.
- J. V. Crivello, Nucl. Instrum. Methods Phys. Res., Sect. B, 1999, 151, 8 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2014 |