Short and efficient synthesis of a daunosamine donor from L-fucal†
Markus Ohlin,
Sophie Manner,
Johanna Löfgren,
Andrea Persson and
Ulf Ellervik*
Center for Analysis and Synthesis, Chemical Center, Lund University, P.O. Box 124, SE-221 00 Lund, Sweden. E-mail: ulf.ellervik@chem.lu.se; Fax: +46 46 2220209; Tel: +46 46 2228220
First published on 20th February 2014
Abstract
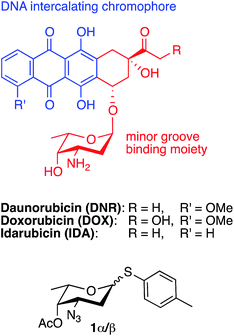
Anthracyclines, e.g. daunorubicin, doxorubicin, and idarubicin, consist of a tetracycline moiety linked via a glycosidic bond to a sugar residue, usually the aminosugar daunosamine. The anthracyclines are efficient chemotherapeutic agents against cancer, but their use is limited due to cardiotoxicity and induction of multidrug resistance. In the search for new anthracycline analogs, a daunosamine donor that can be used to glycosylate suitable aglycons is of utmost importance. Here, we present a short and efficient synthesis of the versatile donor p-tolyl 4-O-acetyl-3-azido-2,3,6-trideoxy-1-thio-α-L-lyxo-hexopyranoside in 3 steps from commercially available L-fucal with an overall yield of 32%. The same procedure can be used to synthesize the donor p-tolyl 4-O-acetyl-3-azido-2,3,6-trideoxy-1-thio-α-L-arabino-hexopyranoside in 28% overall yield from L-rhamnal, for the synthesis of epirubicin analogs.
Introduction
Daunorubicin was isolated from Streptomyces peucetius in the early 1960s and has, together with a few additional anthracyclines, reached the clinical market as an efficient chemotherapeutic agent against cancer.1,2 Anthracyclines consist of a tetracycline moiety linked via a glycosidic bond to a sugar residue, usually the aminosugar daunosamine. The clinically most important anthracyclines are daunorubicin (DNR), doxorubicin (DOX) and idarubicin (IDA) (Fig. 1).The anthracyclines show high affinity for DNA and RNA, and are suggested to act by forming a ternary complex with DNA topoisomerase II and DNA, and thus creating DNA strand breaks, but also by intercalating DNA and by that inhibiting replication and transcription. The detailed mechanisms are not yet known. Unfortunately, the use of anthracylines is limited due to cardiotoxicity and induction of multidrug resistance.1
In the search for new, more effective compounds with moderate general toxicities, a large number of anthracycline analogs have been developed by semi-synthetic methods, although very few have matched the activity of the original compounds. The non-intercalating domain, i.e. the daunosamine sugar and the aliphatic ring, has been shown to be crucial for the ability of the anthracyclines to bind the DNA minor groove and to inhibit DNA topoisomerase.3 So far, most analogs have been variations of the anthraquinone skeleton and there are few examples of truncated compounds.
One problem, in the search for new analogs, has been the lack of versatile daunosamine donors that can be produced in reasonable quantities. In 2007, Fan and coworkers published a valuable daunosamine donor.4 Starting from 3,4-di-O-acetyl-L-rhamnal, a fairly straightforward synthesis in 7 steps gave the daunosamine donor 1α/β (p-tolyl 4-O-acetyl-3-azido-2,3,6-trideoxy-1-thio-α,β-L-lyxo-hexopyranoside) in an overall yield of 21% (α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β 2
β 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The donor was used in the synthesis of daunorubicin analogs with truncated daunomycinone aglycones, i.e. tetrahydro-naphthalene or tetrahydro-anthracene derivatives. The thioglycoside donors were activated with silver hexafluorophosphate and used for α-selective glycosylations in good yields. Finally, the azide moieties were converted to the corresponding amines by Staudinger reduction, and thereby proving the usefulness of the donor.4
1). The donor was used in the synthesis of daunorubicin analogs with truncated daunomycinone aglycones, i.e. tetrahydro-naphthalene or tetrahydro-anthracene derivatives. The thioglycoside donors were activated with silver hexafluorophosphate and used for α-selective glycosylations in good yields. Finally, the azide moieties were converted to the corresponding amines by Staudinger reduction, and thereby proving the usefulness of the donor.4
Here we report a short and efficient synthesis of 1α starting from commercially available 3,4-di-O-acetyl-L-fucal (3,4-di-O-acetyl-1,5-anhydro-2-deoxy-L-lyxo-hex-1-enitol, 2).5 The rationale for using 2 as starting material for the daunosamine donor is the correct stereochemistry of two of the three relevant stereocenters (i.e. C-4 and C-5).
Results and discussion
We explored three different routes from 2 to 1, all based on the Ferrier type 1 reaction, i.e. the addition of nucleophiles to the anomeric position to yield 2,3-unsaturated glycosides (Schemes 1 and 2). | ||
| Scheme 1 Attempted routes A and B, reagents and conditions: (a) NaN3, BF3·OEt2, CH3CN, r.t., 2 h. (b) HSTol, BF3·OEt2, PhMe, 0 °C to r.t., 4 h, 44%. | ||
It is known that glycals can react with sodium azide/BF3·OEt2 in acetonitrile to give enopyranosyl azides, in a standard Ferrier type 1 reaction (Scheme 1, route A). The enopyranosyl products then undergo [3,3] sigmatropic rearrangements to give 3-azido-3-deoxy glycals. Usually the 3-azido regioisomer is the major product under thermodynamic control.6–9 Even though the regioisomeric distribution can be predicted for a variety of glycals (D-glucal, D-allal, L-rhamnal and D-xylal), the stereochemical outcome is less predictable. In 1980, Guthrie and coworkers showed that additions to tri-O-acetyl-D-allal and -D-glucal gave similar product distributions, i.e. a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of axial
1 ratio of axial![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) equatorial azide.7 Later, Paulsen et al. described a similar reaction using D-xylal which further favored the axial product, i.e. a 4
equatorial azide.7 Later, Paulsen et al. described a similar reaction using D-xylal which further favored the axial product, i.e. a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of axial
1 ratio of axial![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) equatorial azide.8 To the best of our knowledge there are no examples of this reaction with L-fucal and we thus subjected 2 to these conditions, aiming for 4. To our surprise, no 3-azido-3-deoxy fucal (4) was formed. We speculate in that the enopyranosyl azide (3) is the preferred product in this case.
equatorial azide.8 To the best of our knowledge there are no examples of this reaction with L-fucal and we thus subjected 2 to these conditions, aiming for 4. To our surprise, no 3-azido-3-deoxy fucal (4) was formed. We speculate in that the enopyranosyl azide (3) is the preferred product in this case.
Next, we evaluated the possibility of reversing the reaction sequence i.e. introducing the thiocresyl (p-methyl-thiophenyl) moiety prior to the azido functionality (Scheme 1, route B). By using thiocresol as nucleophile in the first Ferrier type 1 reaction we isolated the thiocresyl pseudo fucal 5 in moderate yield (44%).10 Further on, we explored conditions for introduction of the azido functionality: NaN3/HOAc/H2O, NaN3/pTSA/HOAc, NaN3/TFA/DCM, TMSN3/TFA/DCM, and AgN3/HBr/HOAc/DMF. Unfortunately, none of these reaction conditions gave the desired product. The C2–C3 double bond is seemingly inert toward mild acidic conditions. Under stronger acidic conditions, the material decomposed. In contrast, the addition of azide ions to the corresponding pseudo fucal (6, vide infra) worked well. We speculate that this reaction is limited to unsaturated hemiacetals, where the open form constitutes an α,β-unsaturated aldehyde intermediate that can be attacked by the nucleophile, in a Michael addition reaction.
Finally, we decided to test a protocol similar to the one used by Florent11 and Fan (Scheme 2, route C).4 Thus, we formed the corresponding pseudo fucal 6 by reaction in water at 80 °C for 3 h. The pseudo fucal was not isolated but instead subjected to NaN3/AcOH/H2O in r.t. for 24 h to give the hemiacetal 7, which was subsequently acetylated using standard conditions (pyridine/acetic anhydride). The reaction mixture was coevaporated with toluene to get crude 8.
Initial glycosylation attempts of compound 8 towards donor 1α/β using BF3·OEt2 at r.t. resulted in a complicated mixture of products. Apart from 1α, we also isolated compound 9 (Chart 1). This material is most probably formed by internal transfer of the anomeric acetyl group. To avoid the formation of 9, the temperature was lowered to −40 °C and the amounts of thiocresol and BF3·OEt2 were decreased to 1.2 and 1.25 equivalents, respectively. In addition, the reaction time was shortened and the reaction was quenched after 5 minutes. Consequently, fewer side-products were formed, which simplified the purification. In addition, during these conditions, the glycosylation was found to be highly α-selective and only trace amounts of 1β was detected.
With optimized glycosylation conditions we returned to the Ferrier reaction. The problems seemed to be long reaction times, with associated decomposition of the product. Thus, we turned to microwave heating and performed the first step, i.e. the formation of the pseudo fucal 6, in a microwave reactor at 100 °C for 15 min, followed by addition of NaN3/HOAc, and further microwave heating for 10 minutes to obtain 7. The crude reaction mixture was worked up, acetylated and glycosylated to give 19% of 1 over three steps. Unfortunately this material was contaminated with a small amount of compound 5, which could not be separated from 1. The presence of 5 indicated that the addition of azide to 6 was incomplete, and therefore we prolonged the reaction times. As expected, after 30 minutes, no traces of 5 could be observed, but the overall yield was lowered to 16%, mainly due to decomposition. Next, we tried a one-pot reaction where compound 2 was dissolved in H2O/HOAc/NaN3 and heated in a microwave reactor at 100 °C. With a total reaction time of 20 minutes, the conversion was raised to 22% but accompanied by a substantial amount of 5 (ratio 1 to 5 was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15). Without acid catalysis (acetic acid) the ratio 1
0.15). Without acid catalysis (acetic acid) the ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 was further increased to 1
5 was further increased to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.64. Finally, we increased the amount of NaN3 from 2 to 4 or 6 equivalents, which gave compound 1α in 30% and 32% yield respectively, without any trace of 5.
0.64. Finally, we increased the amount of NaN3 from 2 to 4 or 6 equivalents, which gave compound 1α in 30% and 32% yield respectively, without any trace of 5.
Under these optimized conditions we could only detect trace amounts of β-glycosides (1β or 10β, Chart 1). However, the addition of the azide ion to the pseudo fucal is not satisfactorily stereoselective and our reaction conditions gave a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (1α
1 ratio (1α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10α). In a similar reaction sequence starting from 6, generated in situ, using 1.75 equivalents of NaN3 in THF at r.t. for 10 hours, Renneberg et al. observed a 1
10α). In a similar reaction sequence starting from 6, generated in situ, using 1.75 equivalents of NaN3 in THF at r.t. for 10 hours, Renneberg et al. observed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4 ratio (equatorial
2.4 ratio (equatorial![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) axial) in the addition of azide.12 Shorter reaction times and elevated temperatures seemingly reverses the selectivity.
axial) in the addition of azide.12 Shorter reaction times and elevated temperatures seemingly reverses the selectivity.
To broaden the scope of this synthetic procedure, we isolated the corresponding donor for epirubicin, i.e. p-tolyl 4-O-acetyl-3-azido-2,3,6-trideoxy-1-thio-α-L-arabino-hexopyranoside4 (12α) in 28% overall yield from L-rhamnal (Scheme 3).
Our optimized synthetic procedure is only limited by the microwave reactor size. We have run the reaction in a 2 g scale with similar yields.
Conclusions
In summary, we have investigated three different routes to the versatile daunosamine donor 1α. In the optimized procedure, 1α can be produced in three synthetic steps with one final chromatographic separation in an overall yield of 32%. The synthetic sequence can be performed in one day. The donor 12α was synthesized in 28% overall yield from L-rhamnal, using the same procedure.Experimental
General methods
Known and commercially available compounds were in agreement with previously published data (NMR). Anhydrous DCM was available via a solvent dispensing system (MB SPS-800). Moisture sensitive reactions were carried out under N2 using dried glassware. NMR spectra were recorded on a Bruker Avance II at 400 MHz (1H) and at 100 MHz (13C), operating at 294 K. Chemical shifts are given in ppm downfield from Me4Si, with reference to residual CHCl3, 7.26 ppm. Reactions were monitored by TLC using alumina plates coated with silica gel (Merck 60 F254) and visualized using either UV light or by charring with ethanolic H2SO4 or staining with a solution of p-methoxybenzaldehyde (26 mL), glacial acetic acid (11 mL), concentrated H2SO4 (35 mL) and 95% ethanol (960 mL). Microwave heated reactions were performed in sealed tubes with a Biotage™ Initiator Classic microwave instrument using an external surface temperature sensor. Preparative chromatography was performed with an automated Biotage™ Isolera One purification apparatus.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 A–B (23 CV), linear gradient to 95
0 A–B (23 CV), linear gradient to 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 A–B (16 CV), 95
5 A–B (16 CV), 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 A–B (14 CV) where A: petroleum ether and B: Et2O; flow-rate: 50 mL min−1; UV detection at 254 nm) to give 1α (241 mg, 32%). Analytical data were in agreement with previously published results.4
5 A–B (14 CV) where A: petroleum ether and B: Et2O; flow-rate: 50 mL min−1; UV detection at 254 nm) to give 1α (241 mg, 32%). Analytical data were in agreement with previously published results.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 A–B (10 CV), linear gradient to 95
0 A–B (10 CV), linear gradient to 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 A–B (110 CV), where A = heptane and B = EtOAc; flow-rate = 25 mL min−1; UV detection at 254 nm) to give 5 (74 mg, 44%), as a white solid. [α]20D +0.03 (c 1.5, CHCl3). 1H NMR (CDCl3): δ 7.41, 7.11 (ABq, 2H each, J = 16.0 Hz, Ar), 6.20 (ddd, 1H, J = 9.9, 3.4, 0.4 Hz, H-2), 6.05 (ddd, 1H, J = 9.8, 5.4, 1.7 Hz, H-3), 5.76 (dd, 1H, J = 3.3, 1.7 Hz, H-1), 5.03 (dd, 1H, J = 5.5, 2.5 Hz, H-4), 4.54 (dq, 1H, J = 6.6, 2.4 Hz, H-5), 2.33 (s, 3H, Ar-CH3), 2.10 (s, 3H, CO2CH3), 1.27 (d, 3H, J = 6.8 Hz, H-6). 13C NMR (CDCl3): δ 170.7, 137.6, 131.9, 131.6, 131.5, 129.8, 125.0, 84.2, 65.7, 65.4, 21.2, 21.0, 16.1. HRMS calcd for C15H18O3SNa (M + Na): 301.0874; found: 301.0878.
5 A–B (110 CV), where A = heptane and B = EtOAc; flow-rate = 25 mL min−1; UV detection at 254 nm) to give 5 (74 mg, 44%), as a white solid. [α]20D +0.03 (c 1.5, CHCl3). 1H NMR (CDCl3): δ 7.41, 7.11 (ABq, 2H each, J = 16.0 Hz, Ar), 6.20 (ddd, 1H, J = 9.9, 3.4, 0.4 Hz, H-2), 6.05 (ddd, 1H, J = 9.8, 5.4, 1.7 Hz, H-3), 5.76 (dd, 1H, J = 3.3, 1.7 Hz, H-1), 5.03 (dd, 1H, J = 5.5, 2.5 Hz, H-4), 4.54 (dq, 1H, J = 6.6, 2.4 Hz, H-5), 2.33 (s, 3H, Ar-CH3), 2.10 (s, 3H, CO2CH3), 1.27 (d, 3H, J = 6.8 Hz, H-6). 13C NMR (CDCl3): δ 170.7, 137.6, 131.9, 131.6, 131.5, 129.8, 125.0, 84.2, 65.7, 65.4, 21.2, 21.0, 16.1. HRMS calcd for C15H18O3SNa (M + Na): 301.0874; found: 301.0878.Acknowledgements
This work was supported by grants from the Swedish Research Council, The Royal Physiographic Society in Lund, and Lund University.Notes and references
- G. Minotti, P. Menna, E. Salvatorelli, G. Cairo and L. Gianni, Pharmacol. Rev., 2004, 56, 185 CrossRef CAS PubMed
.
- L. Zhu, X. Cao, W. Chen, G. Zhang, D. Sun and P. G. Wang, Bioorg. Med. Chem., 2005, 13, 6381 CrossRef CAS PubMed
.
- F. Zunino, G. Pratesi and P. Perego, Biochem. Pharmacol., 2001, 61, 933 CrossRef CAS
.
- E. Fan, W. Shi and T. L. Lowary, J. Org. Chem., 2007, 72, 2917 CrossRef CAS PubMed
.
- Compound 2 can easily be synthesized from peracetylated fucose by formation of the bromosugar followed by treatment with zinc metal in a phosphate buffer. J. Zhao, S. Wei, X. Ma and H. Shao, Carbohydr. Res., 2010, 345, 168 CrossRef CAS PubMed
.
- K. Heyns, M. Lim and J. I. Park, Tetrehedron Lett., 1976, 17, 1411 Search PubMed
.
- R. D. Guthrie and R. W. Irvine, Carbohydr. Res., 1980, 82, 207 CrossRef CAS
.
- H. Paulsen and H. Patt, Liebigs Ann. Chem., 1981, 1633 CrossRef CAS
.
- J. Jaunzems, D. Kashin, A. Schönberger and A. Kirschning, Eur. J. Org. Chem., 2004, 16, 3435 CrossRef
.
- L. V. Dunkerton, N. K. Adair, J. M. Euske, K. T. Brady and P. D. Robinson, J. Org. Chem., 1988, 53, 845 CrossRef CAS
.
- J.-C. Florent and C. Monneret, J. Chem. Soc., Chem. Commun., 1987, 1171 RSC
.
- B. Renneberg, Y.-M. Li, H. Laatsch and H.-H. Fiebig, Carbohydr. Res., 2000, 329, 861 CrossRef CAS
.
- Y. Bai, S.-J. Lin, G. Qi, M. C. Palcic and T. L. Lowary, Carbohydr. Res., 2006, 341, 1702 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of 1H and 13C NMR spectra. See DOI: 10.1039/c4ra01056f |
| This journal is © The Royal Society of Chemistry 2014 |