Preparation and characterization of highly sodium ion conducting Na3PS4–Na4SiS4 solid electrolytes
Abstract
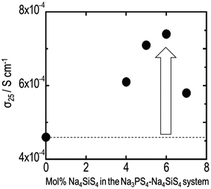
This study investigated electrical and electrochemical properties of (100 − x)Na3PS4·xNa4SiS4 (mol%) glass–ceramics prepared using mechanical milling and consecutive heat treatment. Glass–ceramics at the compositions of 0 ≤ x ≤ 10 exhibited higher conductivity than 10−4 S cm−1 at room temperature, which was achieved by precipitation of cubic Na3PS4 crystal with high sodium ion conductivity. The conductivity increased concomitantly with increasing Na4SiS4 contents at compositions of 0 ≤ x ≤ 6. The glass–ceramic with 6 mol% Na4SiS4 exhibited conductivity of 7.4 × 10−4 S cm−1 at room temperature, which is the highest value reported for Na+ ion conducting sulfides to date. The conductivities of the glass–ceramics in the composition range of 25 ≤ x ≤ 100, where unknown phases are crystallized, were 10−7–10−5 S cm−1. These were lower than the conductivities of the corresponding glasses before heat treatment. The 90Na3PS4·10Na4SiS4 electrolyte showed a wide electrochemical window of 5 V.

 Please wait while we load your content...
Please wait while we load your content...