A novel fuel cell membrane with high efficiency†
Abstract
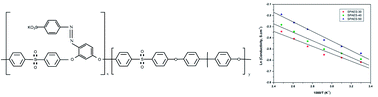
A new class of polymer electrolyte membranes containing an azo based ionic diol (30, 40 and 50 mol%) was prepared for use in a fuel cell. Its proton conductivity, hydrolytic stability, water uptake capacity, swelling behaviour and ion-exchange capacity measurement data indicated its suitability for use as a proton exchange electrolyte membrane in fuel cells. For example, the membranes containing 30, 40 and 50 mol% of azo based ionic monomers exhibit fairly good proton conductivities of 0.073 S cm−1, 0.075 S cm−1 and 0.079 S cm−1, respectively at 30 °C. Moreover, the prepared membranes show a phase separated morphology and exhibit a high thermal stability up to 460 °C which are important parameters for successful fuel cell design. Two types of azo based sulfonated poly(arylene ether sulfone) (SPAES) such as the SPAES-30 and SPAES-50 membrane electrode assembly have been successfully fabricated and yielded a good fuel cell performance in the whole range of current density.

 Please wait while we load your content...
Please wait while we load your content...