Core–shell polysiloxane–MOF 5 microspheres as a stationary phase for gas–solid chromatographic separation†
Manjuab,
Prasun Kumar Roy*a,
Arunachalam Ramanan*b and
Chitra Rajagopala
aCentre for Fire, Explosive and Environment Safety, DRDO, Delhi-54, India. E-mail: pk_roy2000@yahoo.com
bDepartment of Chemistry, Indian Institute of Technology Delhi, New Delhi-16, India. E-mail: aramanan@chemistry.iitd.ac.in
First published on 1st April 2014
Abstract
Core–shell poly(dimethylsiloxane) (PDMS)–MOF 5 microspheres were prepared by directed crystallization of MOF 5 on thermally stable PDMS beads. The microspheres were evaluated for their potential use as a stationary phase for gas-chromatographic separation of permanent gases and liquids, where the issues associated with pressure drop were circumvented. The successful demonstration of this simple and versatile methodology widens the scope for large-scale application of Metal–Organic Frameworks (MOFs) in chromatographic separation.
1. Introduction
In view of their intriguing structures, exceptionally high surface areas, selective adsorption and high thermal stability, metal–organic frameworks (MOFs) present immense potential in various fields, particularly for gas storage and separation.1–4 The crystalline network formed by the self assembly of metal ions and multidentate organic linkers presents ample scope in terms of in-pore functionalization and outer-surface modification to enable favourable interactions based on both “molecular sieving” as well as “chemical affinity” rendering these materials ideal for gas–liquid mixture separation. Lately numerous studies illustrating the potential of MOFs towards gas chromatographic separations have been reported;5 but have not been exploited at an industrial level.Efficient chromatographic separation in packed columns require the particle size of stationary phase to be large enough to circumvent issues associated with pressure drops (see ESI†). The pressure drop in a packed column is inversely proportional to the particle size, and it becomes increasingly impractical to drive the carrier gas in columns containing smaller particles. Early studies on MOF based chromatographic separations on packed beds relied on large crystals6–8 or MOF pellets9 in relatively short column (2–5 cm). To evade the pressure drop issue, researchers have lately directed their attention towards MOF coated capillary columns which additionally result in efficient separations.4,10,11 However, these columns are rather fragile, and suffer from their inherent problems, arising from uncontrolled film formation on the inner capillary walls. Also, capillary columns require relatively specialized injectors and ancillary flow and pressure controllers. Unfortunately, large scale synthesis of MOFs can only afford polycrystalline materials, which cannot be directly packed in chromatographic columns for reasons mentioned above. To envisage economically viable chromatographic applications of functional MOFs at an industrial level, it is desirable to develop methodologies or processes for integrating smaller crystallites that are amenable for scale-up.
We hypothesise that efficient MOF based separations can very well be performed on conventional packed beds by immobilizing the MOFs on an inert template so as to increase the particle size suitably for use in chromatographic columns. The core material has been selected from the broad group of siloxanes, in view of their excellent thermal stability and their widespread use as a template in the field of soft lithography,12 and MOF 5 was selected based on its celebrated nature. The crystal structure of Zn4(O)(BDC)3 or MOF 5 framework is made of oxocentered Zn4O connected through linear benzenedicarboxylate (BDC) units forming an extended 3D cubic network with interconnected pores of 8 Å aperture width and 12 Å pore diameter (Fig. 1). Inset shows oxo-centered Zn4O tetrahedra connected through linear BDC linkers creating open pores.
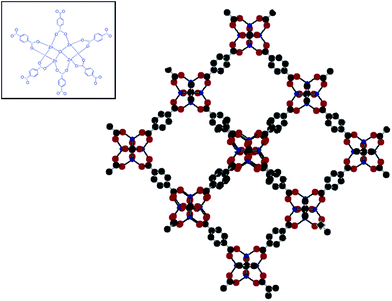 | ||
| Fig. 1 Secondary building unit and crystal structure of MOF 5 (Zn: blue; C: grey; H: white and O: red). | ||
In this paper, we report a simple procedure for directed crystallisation of MOF 5 on the PDMS microspheres, which have been subsequently employed as a stationary phase for gas chromatographic separations. This route yields particles with core–shell morphology requiring substantially lesser amount of MOFs, which is expected to result in lesser band broadening in the chromatograms compared to fully porous materials, thereby delivering higher efficiencies. The methodology can be applied to prepare any PDMS (core)–MOF (shell) combination, and can be used to generate novel stationary phase for chromatographic separations.
2. Materials and methods
2.1. Starting materials
Silicone resin (Elastosil M4644) and the platinum based hardener was obtained from Wacker, Germany. PVA (mol. wt 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, CDH), zinc nitrate trihydrate (‘AR’ grade, E. Merck), terephthalic acid (TPA) (‘AR’ grade, E. Merck), N,N-dimethylformamide (DMF) (‘AR’ grade, E. Merck) and chloroform (CDH) were used without any further purification. Distilled water was used throughout the course of study.
000, CDH), zinc nitrate trihydrate (‘AR’ grade, E. Merck), terephthalic acid (TPA) (‘AR’ grade, E. Merck), N,N-dimethylformamide (DMF) (‘AR’ grade, E. Merck) and chloroform (CDH) were used without any further purification. Distilled water was used throughout the course of study.
2.2. Preparation of core–shell PDMS–MOF-5 microspheres
The suspension curing of vinyl terminated methyl hydrosiloxane dimethylsiloxane at 45 °C in the presence of a hydrosilylation catalyst was performed as per the procedure reported earlier.13,14 The PDMS core was employed as a seed for directed crystallization of MOF 5 as per the procedure reported previously.15 In brief, separate solution of terephthalic acid (TPA 5.06 g, 30.5 mmol) and zinc acetate dihydrate (16.99 g, 77.4 mmol) were introduced into a suspension of PDMS (10 g) in DMF, and allowed to react for ∼2.5 h under stirring at 600 rpm, which resulted in microspheres with core–shell morphology.2.3. Characterization
The identification of crystalline phases in the sample was performed by powder X-ray diffraction (PXRD) analysis on Bruker D8 advanced diffractometer using Nickel filter Cu-Kα radiation. The data was collected with a step size of 0.02° and at count time of 1 s per step over the range of 2–60° (2θ value). The effect of MOF-5 loading on the microsphere dimensions was determined by a particle size analyser (DIPA 2000, Donner). Fourier Transform Infra-Red (FTIR) spectra of samples were recorded in the wavelength range 4000–600 cm−1 using a Thermo Fisher FTIR (NICOLET 8700) analyser with an attenuated total reflectance (ATR) crystal accessory. The textural properties of microspheres were determined by N2 adsorption–desorption on a Surface Area Analyzer (Micromeritics ASAP 2020). For this purpose, the sample was initially out gassed under vacuum (10−6 Torr) at 200 °C for 16 h and the nitrogen adsorbate was pulsed at 77 K. Surface area was calculated from the linear part of the Brunauer–Emmett–Teller (BET) plot and Barrett–Joyner–Halenda (BJH) method was applied on the nitrogen desorption data to determine the pore size distribution. Thermal degradation behaviour was investigated using Perkin Elmer Diamond STG-DTA-DSC under N2 atmosphere in the temperature range of 50–800 °C. A heating rate of 10 °C min−1 and a sample mass of 5.0 ± 0.5 mg were used for each experiment. The surface morphology of samples was studied using a Scanning Electron Microscope (SEM) (Zeiss EVO MA15) under an acceleration voltage of 20 kV. Samples were mounted on aluminium stubs and coated with gold and palladium (10 nm) using a sputter coater (Quorum-SC7620) operating at 10–12 mA for 120 s. The core–shell structure was confirmed using Energy Dispersion Analyser (EDS).2.4. Evaluation of core–shell microspheres for gas separation
The MOF 5 loaded PDMS microspheres were sieved to obtain particles of 60–80 mesh BSS (177–250 μm), which were packed in a stainless steel column (2 m length × 0.025 m diameter) for evaluation of its efficiency towards separation of gaseous and liquid mixtures. A gas chromatograph (NUCON, India), equipped with Thermal Conductivity Detector (TCD) and Flame Ionization Detector (FID) was used for detection of the gaseous eluents. The prepared column was pre-activated under a continuous flow of argon at 200 °C for 24 h. Standard mixture of gases and liquids were injected into the column and the response of TCD and FID was recorded to generate the gas chromatogram.3. Results and discussion
In this paper, we report a simple procedure for preparation of core–shell poly(dimethylsiloxane)–MOF 5 microspheres, which have been subsequently employed as a stationary phase for gas chromatographic separations.3.1. Suspension curing of siloxane
Suspension polymerisation of vinyl terminated methyl hydrosiloxane dimethylsiloxane, in the presence of a hydrosilylation catalyst led to the formation of smooth PDMS beads.13 The effect of operating parameters on the particle dimensions is presented in Fig. S1 (ESI†), which reveal that the particle size distribution shifts towards larger sized microspheres with increasing polymerisable content in the dispersed oily droplets. In all cases, complete conversion (>98%) was achievable, as evidenced by gravimetric analysis.3.2. Effect of MOF-5 loading on the microsphere dimensions and morphology
The surface morphology of PDMS core and core–shell microspheres as revealed by Scanning Electron Microscopy (SEM) are presented in Fig. 2a and b. Magnified image of MOF 5 crystallites on the surface are also presented in the inset of Fig. 2b. The presence of MOF 5 on the surface of core–shell microspheres was further confirmed by EDS analysis, which indicate the presence of zinc in the shell region of the microsphere.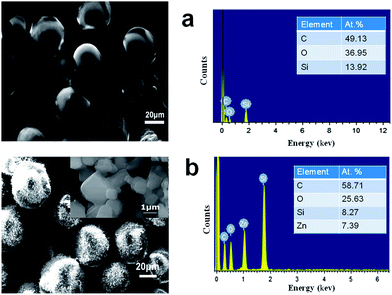 | ||
| Fig. 2 Surface morphology and elemental analysis of (a) PDMS and (b) core–shell PDMS–MOF 5 microspheres. Inset shows the magnified image of MOF 5 crystallites on the surface. | ||
The BET surface area was determined by physisorption of N2 at 77 K. The adsorption–desorption isotherms of the MOF 5 loaded microspheres and the core PDMS are presented in Fig. 3. PDMS microspheres exhibited non-porous nature as evidenced by negligible nitrogen uptake and low surface area (8 m2 g−1). The loading of MOF 5 on the core PDMS resulted in a tremendous increase in the surface area (2850 m2 g−1) and the corresponding adsorption isotherms revealed its characteristic microporous nature. To evaluate the adherence of the MOF 5 coating onto the core PDMS, the MOF 5 loaded microspheres were soaked in DMF for 30 min, followed by exposure to ultrasonic water bath (operating frequency 33 kHz) for 15 min. The coating adhered strongly, with the extent of mass loss being negligible (<2%), as estimated gravimetrically.
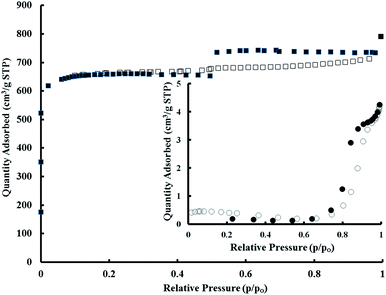 | ||
| Fig. 3 N2 adsorption (open symbol) and desorption isotherms (filled symbol) for PDMS–MOF 5. Inset shows the adsorption–desorption isotherms of core PDMS. | ||
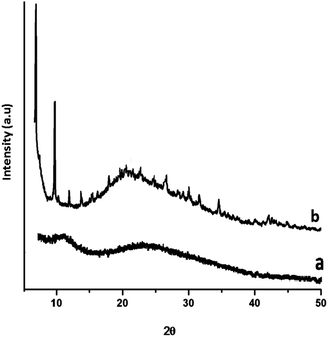
The PXRD pattern of the siloxane core and PDMS–MOF 5 is presented in Fig. 4, which clearly reveals the amorphous nature of PDMS. On the other hand, the XRD of MOF 5 loaded PDMS exhibit distinct diffraction peaks, and the peak positions match with the powder pattern generated by Crystallographic Information File (CCDC-277428).
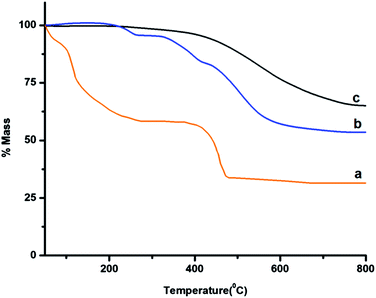
The TGA traces of the siloxane core, MOF 5 and core–shell microspheres (PDMS–MOF 5) are presented in Fig. 5, which reveal that prepared microspheres exhibit excellent thermal stability and can be used in service till 250 °C.
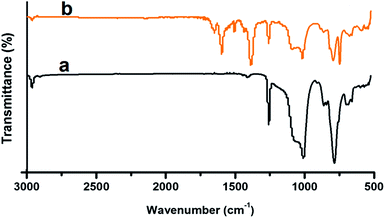
The FTIR of the PDMS, both before and after MOF loading is presented in Fig. 6. In the FTIR spectra of PDMS, characteristic absorption at 802 and 1258 cm−1 were observed which could be attributed to the (CH3)2SiO group vibration in the polymer. Additionally a broad absorption at 1000–1130 cm−1 was also observed, which can be attributed to the Si–O–Si vibration. Due to the coordination of the carboxylic acid groups with the metal ions, there is a significant shift in the position of the CO absorption band, from 1676 cm−1, in TPA, to 1657 cm−1 in MOF 5 (Fig. 6b). Coordination of the linker with the metal ions leads to the disappearance of the CO absorption band at 1281 cm−1 and the broad absorption due to the hydroxyl groups ∼3000–3200 cm−1.
3.3. Gas-chromatographic separations
A standard mixture (50 μL) of permanent gases in 59.03% H2, 20.05% N2, 5.05% CH4, 10.0% CO2 and 5% CO, (% v/v) (Sigma gases) was injected into the GC column and the chromatogram in terms of the detector response is presented in Fig. 7.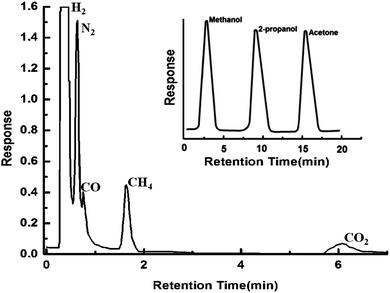 | ||
| Fig. 7 Gas chromatograms showing effective separations of H2, N2, CO, CH4 and CO2. Inset shows the gas chromatogram exhibiting excellent separation of liquids. | ||
The retention time of each component, as determined by injection of individual gas separately, is given in the ESI (see Table S1, ESI†). The order of elution of the individual gases from the column is in accordance with increasing order of their kinetic diameters (H2, 2.89 Å; N2, 3.64 Å; CO, 3.76 Å; CH4, 3.8 Å and CO2, 3.3 Å). It is to be noted that the estimated pore size of MOF 5 is too large (∼9 Å) to permit separations based on molecular sieving, and hence the sequence of elution is very well expected. Interestingly, CO2 with a kinetic diameter of 3.3 Å elutes at the end, which can be explained on the basis of strong interaction of CO2 with the MOF 5 framework.16 The efficiency of the packed PDMS–MOF 5 column towards separation of liquids was also established by injecting an equimolar mixture of methanol, propanol and acetone which were detected using flame ionisation technique. The chromatogram (response of FID) as shown in the inset of Fig. 7, clearly establishes the ability of the column towards separation of liquid mixtures. Since the kinetic diameter of all the molecules is lesser than the pore size of MOF-5, the order of elution is in line.
For comparison purpose, chromatographic separations were also performed on commercially available packed columns generally employed for separation of similar gas mixtures, namely molecular sieve 5A (MS 5) and Porapak N. The chromatograms obtained are shown in the ESI.† Carbon dioxide does not elute from MS 5 column due to its strong interactions with the microporous aluminosilicate structure, while Porapak N is incapable of separating H2 and N2 mixture.17 It can be seen that neither of these columns individually could separate all the components, however a 2D separation can be used for effective separations.
The prepared column was also employed to separate a natural gas mixture comprising of 79.37% methane, 6.72% ethane, 0.94% iso-butane, 0.53% iso-pentane, 1.31% N-butane, 0.19% N-hexane, 0.51% N-pentane, 8.09% propane and 2.34% CO2 (% v/v). For this purpose, 50 μL of the gas mixture was injected into the GC column and the separation was effected under conditions mentioned in the ESI.† The TCD response is presented in Fig. 8. The retention time of each component, as determined by injection of individual gas separately, is given in the ESI (see Table S2, ESI†). It can be seen that all the components could be effectively separated by the PDMS–MOF 5 column.
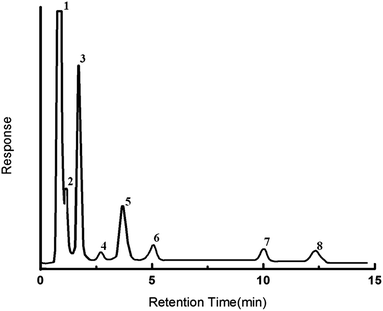 | ||
| Fig. 8 Gas chromatogram showing separation of natural gas mixture. (1) Methane, (2) ethane, (3) propane, (4) carbon dioxide, (5) iso-butane, (6) n-butane, (7) n-pentane, (8) n-hexane. | ||
Our study clearly highlights the potential of MOF loaded microspheres for practical application towards separation of gas mixtures. The reproducibility of the PDMS–MOF 5 column was established by performing repeated runs on gas mixtures obtained from ethanol reforming exit stream,18 comprising primarily of H2, N2, CH4, CO2 and CO, which corroborates excellent separation reproducibility.
It should be noted that in view of tunability of the pore size of MOFs by judicious choice of SBU and linker molecules, it is possible to envisage hitherto unprecedented separations, thereby opening novel opportunities in the field of separation technology.
4. Conclusions
We demonstrate the potential of core–shell PDMS–MOF 5 for effective separation of gas–liquid mixtures in a packed chromatographic column, where the issues associated with pressure drop are circumvented. It should be noted that the methodology reported here could in principle be applied towards crystallisation of any MOF. The ease of this technique suggests scope for wider applicability of MOFs in the field of chromatographic separations.Acknowledgements
The authors are thankful to Dr Sudershan Kumar, Director, Centre for Fire, Explosive and Environment Safety for taking keen interest and for providing the laboratory facilities. The authors also gratefully acknowledge the help extended by Gyan Batra, NUCON, Delhi India, for preparation of the packed SS columns for chromatographic separation.References
- H. Furukawa, N. Ko, Y. B. Go, N. Aratani, S. B. Choi, E. Choi, A. Ö. Yazaydin, R. Q. Snurr, M. O'Keeffe, J. Kim and O. M. Yaghi, Science, 2010, 329, 424–428 CrossRef CAS PubMed.
- S. L. James, Chem. Soc. Rev., 2003, 32, 276–288 RSC.
- U. Mueller, M. Schubert, F. Teich, H. Puetter, K. Schierle-Arndt and J. Pastre, J. Mater. Chem., 2006, 16, 626–636 RSC.
- R. Ahmad, A. G. Wong-Foy and A. J. Matzger, Langmuir, 2009, 25, 11977–11979 CrossRef CAS PubMed.
- C. Montoro, F. Linares, E. Q. Procopio, I. Senkovska, S. Kaskel, S. Galli, N. Masciocchi, E. Barea and J. A. R. Navarro, J. Am. Chem. Soc., 2011, 133, 11888–11891 CrossRef CAS PubMed.
- B. Chen, C. Liang, J. Yang, D. S. Contreras, Y. L. Clancy, E. B. Lobkovsky, O. M. Yaghi and S. Dai, Angew. Chem., Int. Ed., 2006, 45, 1390–1393 CrossRef CAS PubMed.
- S. Han, Y. Wei, C. Valente, I. Lagzi, J. J. Gassensmith, A. Coskun, J. F. Stoddart and B. A. Grzybowski, J. Am. Chem. Soc., 2010, 132, 16358–16361 CrossRef CAS PubMed.
- T. Borjigin, F. Sun, J. Zhang, K. Cai, H. Ren and G. Zhu, Chem. Commun., 2012, 48, 7613–7615 RSC.
- D. Peralta, G. Chaplais, J.-L. Paillaud, A. Simon-Masseron, K. Barthelet and G. D. Pirngruber, Microporous Mesoporous Mater., 2013, 173, 1–5 CrossRef CAS PubMed.
- Z.-Y. Gu, D.-Q. Jiang, H.-F. Wang, X.-Y. Cui and X.-P. Yan, J. Phys. Chem. C, 2009, 114, 311–316 Search PubMed.
- Z.-L. Fang, S.-R. Zheng, J.-B. Tan, S.-L. Cai, J. Fan, X. Yan and W.-G. Zhang, J. Chromatogr. A, 2013, 1285, 132–138 CrossRef CAS PubMed.
- L. Wang, W. Jiang, X. Chen, L. Gu, J. Chen and R. T. Chen, J. Appl. Phys., 2007, 101, 114316–114321 CrossRef PubMed.
- P. K. Roy, M. Hakkarainen and A.-C. Albertsson, Polym. Degrad. Stab., 2012, 97, 1254–1260 CrossRef CAS PubMed.
- P. K. Roy, Manju, C. Rajagopal and A. Ramanan, 2766/DEL/2013, 2013.
- Manju, P. K. Roy, A. Ramanan and C. Rajagopal, Mater. Lett., 2013, 106, 390–392 CrossRef CAS PubMed.
- Z.-Y. Gu and X.-P. Yan, Angew. Chem., Int. Ed., 2010, 49, 1477–1480 CrossRef CAS PubMed.
- J. W. Yoon, S. H. Jhung, Y. K. Hwang, S. M. Humphrey, P. T. Wood and J. S. Chang, Adv. Mater., 2007, 19, 1830–1834 CrossRef CAS.
- P. K. Sharma, N. Saxena, A. Bhatt, C. Rajagopal and P. K. Roy, Catal. Sci. Technol., 2013, 3, 1017–1026 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra00894d |
| This journal is © The Royal Society of Chemistry 2014 |