Layered double hydroxide composite monoliths with three-dimensional hierarchical channels: structural control and adsorption behavior†
Abstract
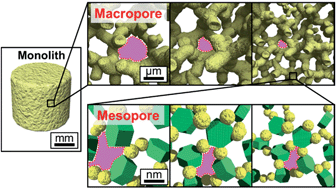
Hierarchically porous layered double hydroxide (LDH) materials have potential in anion-exchange, adsorption and catalysis applications, because of their large surface areas and liquid transportation capabilities. The preparation of monolithic LDH–Al(OH)3 composites with hierarchical μm and nm-scale channels and their adsorption behavior is reported. Monolithic gels were synthesized via sol–gel processing, from metal salt precursor solutions. μm-scale macrochannels spontaneously formed by inducing phase separation during sol–gel transition. nm-scale mesochannels were accommodated as interstices between primary/secondary particles. In this study, these hierarchical channel sizes were controlled. The macrochannel size was controlled by tuning the degree of phase separation. The mesochannel size was controlled independently, by tuning the crystallite size of LDH under different solvothermal conditions. The relationship between pore characteristics and adsorption behavior of tailored hierarchically porous LDH–Al(OH)3 monolithic gels were investigated by using dye molecules as adsorbates. Monolithic gels with larger macrochannels and mesochannels exhibit faster adsorption rate and higher affinity, respectively. LDH–Al(OH)3 monolithic gels with hierarchical channels may have potential in some applications such as biosensing, water purification and catalysis.

 Please wait while we load your content...
Please wait while we load your content...