Fabrication and modification of composite silica nano test tubes for targeted drug delivery†
Abstract
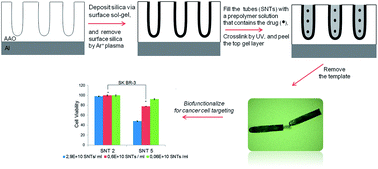
This work describes the use of template synthesis to fabricate multifunctional composite silica nano test tubes for targeted drug delivery. The tubular nanostructures were formed within nanoporous anodized alumina templates and their inner voids were filled with a drug-bearing gel matrix while the test tubes were embedded within the template. Upon template removal, the composite nanocarriers were biofunctionalized with a targeting moiety towards breast cancer cells. The results show that targeting is critical in inducing cell death and the targeted nanocarriers are extensively more cytotoxic towards cancer cells compared with healthy controls.

 Please wait while we load your content...
Please wait while we load your content...