Stereoselective inversion of γ-vinyl-γ-butyrolactone under palladium catalysis: application to the synthesis of (+)-exo- and (+)-endo-brevicomins†
Abstract
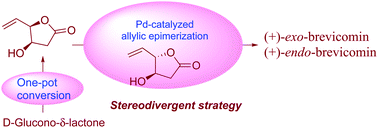
An efficient γ-epimerization of γ-vinyl-γ-butyrolactone is studied under palladium catalysis. The building blocks derived there in are used in an efficient stereoselective synthesis of (+)-exo- and (+)-endo-brevicomins.

 Please wait while we load your content...
Please wait while we load your content...