Improvement of wood material properties via in situ polymerization of styrene into tosylated cell walls†
Abstract
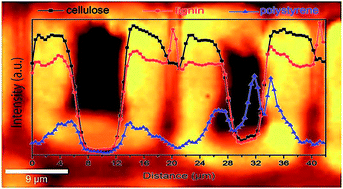
As an engineering material derived from renewable resources, wood possesses excellent mechanical properties in view of its light weight but also has some disadvantages such as low dimensional stability upon moisture changes and low durability against biological attack. Polymerization of hydrophobic monomers in the cell wall is one of the potential approaches to improve the dimensional stability of wood. A major challenge is to insert hydrophobic monomers into the hydrophilic environment of the cell walls, without increasing the bulk density of the material due to lumen filling. Here, we report on an innovative and simple method to insert styrene monomers into tosylated cell walls (i.e. –OH groups from natural wood polymers are reacted with tosyl chloride) and carry out free radical polymerization under relatively mild conditions, generating low wood weight gains. In-depth SEM and confocal Raman microscopy analysis are applied to reveal the distribution of the polystyrene in the cell walls and the lumen. The embedding of polystyrene in wood results in reduced water uptake by the wood cell walls, a significant increase in dimensional stability, as well as slightly improved mechanical properties measured by nanoindentation.

 Please wait while we load your content...
Please wait while we load your content...