Lithium storage improvement from hierarchical double-shelled SnO2 hollow spheres†
Abstract
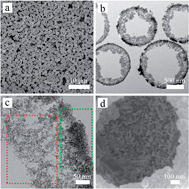
We have developed hierarchical double-shelled SnO2 hollow spheres by a sequential template-engaged method, in which the loosened SnO2 floccules are well-grown on the inside wall of the hollow spheres. The unique hierarchical structure could provide more active sites for Li–Sn alloying–dealloying reaction and fast insertion/extraction of Li+ ion with superb conductivity, helping to store more lithium (2578 mA h g−1) with great cycling stability.

 Please wait while we load your content...
Please wait while we load your content...