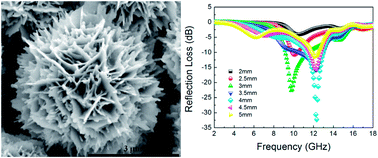
A series of CuS/ZnS nanocomposites (CZS) with a novel 3D hierarchical structure were obtained by a simple hydrothermal method. The effects of Zn2+ content, surfactant, reaction temperature and time on the morphology of the samples was studied. The products were characterized in detail and their photocatalytic performance in the degradation of methylene blue (MB) and microwave absorption properties as an absorber were also investigated. The results indicate that CZS0.4 presented an outstanding photocatalytic degradation rate of more than 97% for MB in the first twenty minutes, and MB was degraded almost completely in the presence of each of the six samples alone 70 minutes after photocatalytic reaction. CZS0.3 exhibited excellent microwave absorption properties, presenting a reflection loss as much as −22.6 dB at 9.7 GHz with a thickness of 3 mm, which is much better than that of pure CuS.