3D high-energy-density and low sensitivity materials: synthesis, structure and physicochemical properties of an azide–Cu(II) complex with 3,5-dinitrobenzoic acid†
Xiangyu Liuab,
Qi Yanga,
Zhiyong Sua,
Sanping Chen*a,
Gang Xiea,
Qing Weia and
Shengli Gaoa
aKey Laboratory of Synthetic and Natural Functional Molecule Chemistry of Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an 710069, China. E-mail: sanpingchen@126.com; Fax: +86-029-88302604; Tel: +86-029-88302604
bSchool of Chemistry and Chemical Engineering, Ningxia University, Yinchuan 750021, China
First published on 6th February 2014
Abstract
A novel 3D energetic coordination polymer of azide–Cu(II), Cu(3,5-DNBA)(N3), was synthesized and structurally characterized by single crystal X-ray diffraction, where 3,5-DNBA represents 3,5-dinitrobenzoic acid. Structural analysis reveals that the central Cu(II) ion coordinates with two azide anions and three 3,5-dinitrobenzoic acid anions to form a five-coordinated tetragonal pyramid structure. Remarkably, one oxygen atom in the nitro group displays rare coordination to the Cu(II) ions in the complex. The as-prepared compound showed abrupt thermal decomposition at 268 °C, representing explosive performance and superior thermostability based on DSC and TG-DTG analyses. Sensitivity tests revealed that the title complex was insensitive to external stimuli. The kinetic parameters of an exothermic process for the complex were studied by Kissinger's and Ozawa–Doyle's methods. In addition, the constant-volume combustion energy of the complex was determined using a precise rotating-bomb calorimeter, and the standard molar enthalpy of combustion and the standard molar enthalpy of formation were calculated.
1. Introduction
Energetic materials include explosives, propellants, and pyrotechnics that are used for a variety of military purposes and civilian applications.1–3 Explosives may be classified as primary and secondary explosives: the former are very sensitive and low-performing compounds,4,5 which are commonly used to initiate a more powerful and less sensitive secondary explosive.6 Unfortunately, high performance and low sensitivity tend to be contradicting aspects.7 In combination with tailored performance, insensitivity, stability, oxygen balance and environmental safety,2,8–10 novel energetic materials have been scarcely reported.11As is already known, Cu(II) exhibits a good coordination ability to different kinds of ligands, and more importantly, is an environmentally-friendly ion compared to heavy metal ions such as lead and mercury.12,13 To date, numerous Cu(II)–nitrogen complexes have been synthesized and characterized in the literature.14 Of particular interest are Cu(II) complexes with nitrogen-rich ligands, which can be used as green energetic materials.15–21 Azide, a simple nitrogen-rich ligand with the highest nitrogen content of 100%, is a prominent energetic moiety which can increase the heat of formation by about 355 kJ mol−1 and can be easily used to alter oxygen balance, and the combustion product of azide is environmentally friendly.2,22,23 Furthermore, as a member of the short bridging ligand group, azide is able to connect transition metal ions via multiple coordination modes. However, for azide–metal complexes, attention is mostly focused on the magnetic properties.24–27 Probably because of the high sensitivity of azide, there is limited literature about the energetic properties of azide–metal complexes at present. Although the literature discloses that the sensitivity of the azide compound depends on the minimum distance between N atoms of adjacent azides,28 how to fine-tune the structures to balance the energy and the sensitivity of azide compounds has been a hot topic and difficulty in the field of energetic materials.
In the present work, we focus our attention on the high-energy of an azide–Cu(II) system. Due to the favorable oxygen balance, these compounds are suitable as energetic fillers in highly explosive compositions.29 Additionally, in order to improve the insensitivity and the stability of the complex, 3,5-dinitrobenzoic acid (3,5-DNBA) is introduced as a co-ligand into the azide–Cu(II) energy system because of its advantages, such as its stable rigid framework, variety of coordination modes, high oxygen content and explosive properties.30,31 In addition, because the sensitivity of an explosive depends on properties such as shear strength and molecular orientations of the crystalline material,32 research into the structure-directing properties for energetic materials has been a topic of permanent interest.
In view of the observations above, we present here the synthesis and crystal structure of a 3D azide–Cu(II) complex, Cu(3,5-DNBA)(N3). Remarkably, an oxygen atom in the nitro group of 3,5-dinitrobenzoic acid connected with Cu(II), which is a rare example in Cu-based energetic coordination compounds in spite of the same coordination action occurring in other metal compounds.33–35 The 3D framework of the complex further benefits by increasing the stability and reducing the sensitivity of the energetic complex but it has no influence on the explosive properties. In addition, thermogravimetric analysis, sensitivity measurements, the thermokinetics and the standard molar enthalpy of formation were investigated.
2. Experimental
General caution: azide, 3,5-DNBA and the title complex are energetic materials and tend to explode under certain conditions. Although no difficulties were experienced in the synthesis of these materials, proper protective measures should be taken, especially when the complex is prepared on a large scale.2.1. Materials and instruments
All reagents (analytical grade) were purchased commercially and used without further purification unless otherwise stated.Elemental analysis was performed using a Vario EL III analyzer fully automated trace element analyzer. The FT-IR spectra were recorded using a Bruker FTIR instrument with the samples as KBr pellets. Differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) were carried out using a Netzsch STA 449C instrument and a CDR-4P thermal analyzer of Shanghai Balance Instrument factory, respectively, using dry oxygen-free nitrogen as the atmosphere, at a flow rate of 10 mL min−1. About 0.5 mg of the sample was sealed in aluminum pans for DSC and a temperature range of 50–600 °C was used. The sensitivity to impact stimuli was determined by fall hammer apparatus, applying a standard staircase method using a 2 kg drop weight and the results were reported in terms of height for 50% probability of explosion (h50%).36 The friction sensitivity of the complex was determined using a Julius Peters apparatus, following the BAM method.37 The phase purity of the bulk sample was verified by X-ray powder diffraction (XRPD) radiation (λ = 1.5406 Å), with a scan speed of 5° min−1 and a step size of 0.02° in 2θ. The constant-volume combustion energy of the complex was determined using an RBC-type II rotating-bomb calorimeter.
2.2. X-ray structure determination
Single crystal X-ray experiments were performed using a Bruker Smart Apex CCD diffractometer equipped with graphite monochromatized Mo Kα radiation (λ = 0.71073 Å) using ω and φ scan modes. The structure was solved by direct methods using SHELXS-97 (ref. 38) and refined by means of full-matrix least-squares procedures on F2 with the SHELXL-97 program.39 All non-H atoms were located using subsequent Fourier-difference methods and refined anisotropically. In all cases hydrogen atoms were placed in calculated positions and thereafter allowed to ride on their parent atoms. Other details of crystal data, data collection parameters and refinement statistics are given in Table 1. Selected bond lengths and bond angles of the title complex are listed in Table S1.† Further information concerning the crystal structure determination in CIF format is available in the ESI.†| Empirical formula | C7H3CuN5O6 |
| Formula weight | 316.68 |
| Crystal system | Orthorhombic |
| Space group | P2(1)2(1)2(1) |
| a (Å) | 6.747(2) |
| b (Å) | 9.047(3) |
| c (Å) | 16.973(5) |
| α (°) | 90 |
| β (°) | 90 |
| γ (°) | 90 |
| V (Å3) | 1036.0(5) |
| Z | 4 |
| μ (mm−1) | 2.145 |
| Unique reflections | 2041 |
| Observed reflections | 5301 |
| Rint | 0.0818 |
| Final R indices [I > 2σ(I)] | R1 = 0.0537, wR2 = 0.1270 |
| R indices (all data) | R1 = 0.0777, wR2 = 0.1408 |
2.3. Synthesis of the complex
A mixture of Cu(NO3)2·3H2O (0.072 g, 0.3 mmol), 3,5-DNBA (0.085 g, 0.4 mmol) and NaN3 (0.013 g, 0.2 mmol) in ethanol (20 mL) were stirred for 1 h at room temperature. After being undisturbed for one week, dark green flake crystals were obtained and collected from the mixture system above. (Yield: 55%, based on Cu) Anal. Calcd. for CuC7H3N5O6 (316.68): C, 26.53; H, 0.95; N, 22.10%. Found: C, 26.81; H, 1.09; N, 22.38%. Main IR (KBr, cm−1): 2048 (s), 1604 (m), 1537 (s), 1500 (m), 1465 (m), 1409 (s), 1272 (w), 1207 (m), 1058 (m), 912 (m), 786 (s).3. Results and discussion
3.1. Structure description
Single-crystal analysis reveals that the complex crystallizes in an orthorhombic system and exists as a 3D network structure. As shown in Fig. 1a, the asymmetric unit contains one crystallographic independent Cu(II), in which Cu(II) is occupied by two nitrogen atoms from two μ-1,1-azide anions [Cu1–N1 = 1.990 Å Cu1–N1A = 1.967 Å], two oxygen atoms of the carboxylic group from two 3,5-DNBA [Cu1–O1 = 1.939 Å, Cu1–O2 = 1.950 Å] and one oxygen atom of the nitro group from another 3,5-DNBA [Cu1–O4 = 2.609 Å] (occupying the apical position). Selected bond lengths and bond angles are shown in Table S1.† The carboxylate group in 3,5-DNBA interacts with Cu(II) in a doubly-bridging fashion, while the nitro is in a monodentate bridging mode (Fig. 1b). Adjacent Cu(II) ions are linked by an azide bridge in μ-1,1 (or end-on, EO) mode and a carboxylate bridge in syn–syn mode, yielding a formal 1D chain along the crystallographic a direction (Fig. 1c). Finally, nitro groups on the intra-chain 3,5-DNBA molecules are connected to Cu(II) ions from other chains, forming a three-dimensional network structure (Fig. 1d). It is noteworthy that one oxygen atom in the nitro group coordinates to Cu(II), which is rare in complexes with nitro groups.33–353.2. Thermal decomposition
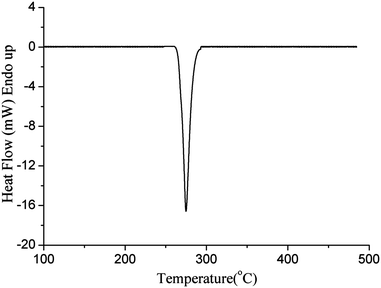
DSC and TG/DTG curves under the linear heating rate of 10 °C min−1 with a nitrogen atmosphere are shown in Fig. 2 and 3 to demonstrate the thermal decomposition processes of powder samples Cu(3,5-DNBA)(N3). In order to confirm the phase purity of the bulk materials, X-ray powder diffraction (XRPD) has been carried out for the powdered sample of the complex (Fig. S1†). The experimental pattern is in good agreement with the corresponding simulated one, indicating the phase purity of the as-synthesized product.In the DSC curve, one intense and sharp exothermic process occurs at 268 °C and ends at 280 °C with a peak temperature of 275 °C (Fig. 2). A typical TG/DTG curve is shown in Fig. 3. A sample size as small as 0.5 mg detonates at 268 °C in an open crucible made of Al2O3. In this case, complete detonation is accompanied by phut and does not destroy the crucible, which may be due to the small quantity of the sample. As noticeable from the TG curve, there is one mass-loss stage, corresponding to only one peak in the DSC curve. A sudden weight loss is observed at 268 °C that stops at 278 °C, accompanied by about 76.1% mass loss, which is attributed to the full decomposition process for the main framework of the complex. The final residue mass is 23.9% of the initial mass, which coincides with the calculated value of CuO (25.3%) determined by XRPD (JPCDS 05-0661). TG analysis shows an explosive performance and good thermal stability of the complex. Additionally, the oxygen balance value is calculated to be −48.0%.
The decomposition temperatures,40 oxygen balance and density values of RDX, HMX, TNT41 and the title complex are summarized in Table 2. Obviously, the density of the complex is bigger than the other explosives, the thermal stability of the complex is better than both RDX and TNT, while the oxygen balance is better than TNT.
| RDX | HMX | TNT | Complex | |
|---|---|---|---|---|
| Formula | C3H6N6O6 | C4H8O8N8 | C7H5N3O6 | C7H3CuN5O6 |
| Molecular mass (g mol−1) | 222.1 | 296.2 | 227.1 | 316.7 |
| ρ (g cm−3) | 1.800 | 1.905 | 1.654 | 2.030 |
| Tdec (°C) | 210 | 282 | 244 | 268 |
| Ω (%) | −21.6 | −21.6 | −74.0 | −48.0 |
3.3. Sensitivity test
Impact sensitivity is determined using Fall Hammer Apparatus. Twenty milligrams of Cu(3,5-DNBA)(N3) is compacted to a copper cap under a 39.2 MPa press, and is hit by a 2 kg drop hammer, and the calculated value of H50 represents the drop height of 50% initiation probability. The test shows that the impact sensitivity value of the complex is 120 cm, which corresponds to an impact energy of 23.5 J. Under the same test conditions, the impact sensitivity value (H50) of TNT is 76.5 cm (15.0 J).42 Consequently, the impact sensitivity of the title complex is lower than that of TNT.The friction sensitivity of the complex is measured by using a Julius Peters machine using 20 mg sample. No friction sensitivity is observed up to 36 kg (360 N). The friction sensitivity of the complex is lower than that of RDX 12 kg.43
The title compound was roughly tested for ESD (Electro-Static Discharge) sensitivity by spraying sparks across a small (5 crystals) sample of the material using a tesla coil. Although it was not confirmed by quantitative testing, the compounds are most likely to be less sensitive to electrostatic discharge than both TNT and RDX.
The results revealed that the title complex is very insensitive to external stimuli due to the structural characteristics of the title complex, which are probably due to the fact that the nitro group is bonded to the metal ion.
3.4. Non-isothermal kinetics
In this work, Kissinger's method44 and Ozawa–Doyle's method45,46 were applied to determine the apparent activation energy (E) and the pre-exponential factor (A). The Kissinger (eqn (1)) and Ozawa–Doyle (eqn (2)) equations are as follows:
 | (1) |
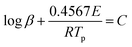 | (2) |
Based on the exothermic peak temperatures measured at four different heating rates of 5, 8, 10, and 15 °C min−1, the thermokinetic parameters of the complex were obtained. The apparent activation energies Ek and Eo, pre-exponential factor Ak and linear correlation coefficients Rk and Ro are shown in Table 3.
| Heating rates (°C min−1) | Peak temperature (°C) |
|---|---|
| 5 | 268.2 |
| 8 | 272.3 |
| 10 | 274.8 |
| 15 | 276.3 |
| The calculated results using Kissinger's method | |
| Ek (kJ mol−1) | 304.2 |
Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ak(s−1) Ak(s−1) |
27.4 |
| Linear correlation coefficient (Rk) | 0.9976 |
| The calculated results using Ozawa–Doyle's method Eo (kJ mol−1) | 297.9 |
| Linear correlation coefficient (Ro) | 0.9968 |
The calculated results using both methods are quite close, which are all in the normal range of kinetic parameters for the thermal decomposition reaction of solid materials.47 The Ea value (301.05 kJ mol−1) for the thermal decomposition stage shows that the exothermic process can not readily proceed. Using the obtained Ea (the average of Ek and Eo) and ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ak values, the Arrhenius equation can be expressed as ln
Ak values, the Arrhenius equation can be expressed as ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k = 27.4 − 301.05 × 103/RT for the exothermic process, which can be used to estimate the rate constant of the initial thermal decomposition process of the title complex.
k = 27.4 − 301.05 × 103/RT for the exothermic process, which can be used to estimate the rate constant of the initial thermal decomposition process of the title complex.
3.5. Standard molar enthalpy of formation for the complex
The methods for the determination and calculation of the constant-volume combustion energy for the complex are the same as those for the calibration of the calorimeter with benzoic acid (SRM, 39i, NIST). The experimental procedures are described in the ESI.† The experimental result is (−10971.11 ± 6.56) J g−1, which has an equivalence of (−3474.33 ± 2.08) kJ mol−1 for the complex.The standard molar combustion enthalpy of the complex, ΔcHθm, refers to the combustion enthalpy change of the following ideal combustion reaction (eqn (3)) at 298.15 K and 101.325 kPa.
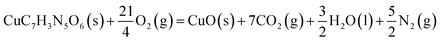 | (3) |
The standard molar combustion enthalpy of the complex is calculated using the following equations.
| ΔcHθm = ΔcU + ΔnRT | (4) |
| Δn = ng (products) − ng (reactants) | (5) |
The standard molar enthalpy of formation of the complex, ΔcHθm, is calculated by Hess's law according to the thermochemical eqn (6).
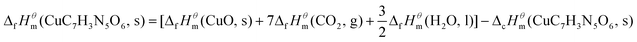 | (6) |
4. Conclusion
A novel three-dimensional azide-bridged Cu(II) coordination polymer, Cu(3,5-DNBA)(N3), has been synthesized and characterized. Remarkably, one oxygen atom in the nitro group coordinates to Cu(II). The result of non-isothermal kinetics analysis shows that the apparent activation energy is 301.05 kJ mol−1. The enthalpy of combustion of the complex is higher than RDX and HMX. The complex shows superior thermostability and insensitivity compared to RDX and TNT. The results above show that the complex is an example of a green, and high energy and insensitivity material.Acknowledgements
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (Grant nos. 21373162, 21203149, 21127004 and 21173168), and the Nature Science Foundation of Shanxi Province (Grant nos. 11JS110, FF10091 and SJ08B09).References
- D. Badgujar, M. Talawar, S. Asthana and P. Mahulikar, Advances in science and technology of modern energetic materials: An overview, J. Hazard. Mater., 2008, 151, 289–305 CrossRef CAS.
- M. Talawar, R. Sivabalan, T. Mukundan, H. Muthurajan, A. Sikder, B. Gandhe and A. S. Rao, Environmentally compatible next generation green energetic materials (GEMs), J. Hazard. Mater., 2009, 161, 589–607 CrossRef CAS.
- B.-D. Wu, Y.-L. Li, F.-G. Li, Z.-N. Zhou, L. Yang, J.-G. Zhang and T.-L. Zhang, Preparation, Crystal Structures and Thermal Decomposition of three Energetic Manganese Compounds and a Salt Based on Imidazole and Picrate, Polyhedron, 2013, 55, 73–79 CrossRef CAS.
- M. B. Talawar, A. P. Agrawal, M. Anniyappan, D. S. Wani, M. K. Bansode and G. M. Gore, Primary explosives: Electrostatic discharge initiation, additive effect and its relation to thermal and explosive characteristics, J. Hazard. Mater., 2006, 137, 1074–1078 CrossRef CAS.
- L. E. Fried and A. J. Ruggiero, Energy transfer rates in primary, secondary, and insensitive explosives, J. Phys. Chem., 1994, 98, 9786–9791 CrossRef CAS.
- S. Meyers and E. S. Shanley, Industrial explosives-a brief history of their development and use, J. Hazard. Mater., 1990, 23, 183–201 CrossRef CAS.
- R.-H. Wang, H.-Y. Xu, Y. Guo, R.-J. Sa and J. M. Shreeve, Bis[3-(5-nitroimino-1,2,4-triazolate)]-based energetic salts: Synthesis and promising properties of a new family of high-density insensitive materials, J. Am. Chem. Soc., 2010, 132, 11904–11905 CrossRef CAS.
- G. Steinhauser and T. M. Klapötke, Green Pyrotechnics: A Chemists' Challenge, Angew. Chem., Int. Ed., 2008, 47, 3330–3347 CrossRef CAS.
- U. R. Nair, S. N. Asthana, A. S. Rao and B. R. Gandhe, Advances in High Energy Materials (Review Paper), Def. Sci. J, 2010, 60, 137–151 CrossRef CAS.
- L. Türker and S. Variş, A review of polycyclic aromatic energetic materials, Polycyclic Aromat. Compd., 2009, 29, 228–266 CrossRef.
- S.-H. Li, Y. Wang, C. Qi, X.-X. Zhao, J.-C. Zhang, S.-W. Zhang and S.-P. Pang, 3D Energetic Metal–Organic Frameworks: Synthesis and Properties of High Energy Materials, Angew. Chem., Int. Ed., 2013, 52, 1–6 CrossRef.
- J. Köhler and J. Meyer, Explosivstoffe, Wiley-VCH, D-Weinheim, 9th edn, 1998, vol. 174 Search PubMed.
- K. Karaghiosoff, T. M. Klapötke, A. N. Michailovski, H. Nöth, M. Suter and G. Holl, 1,4-Diformyl-2,3,5,6-Tetranitratopiperazine: A New Primary Explosive Based on Glyoxal, Propellants, Explos., Pyrotech., 2003, 28, 1–6 CrossRef CAS.
- R. P. Singh, R. D. Verma, D. T. Meshri and J. M. Shreeve, Energetic Nitrogen-Rich Salts and Ionic Liquids, Angew. Chem., Int. Ed., 2006, 45, 3584–3601 CrossRef CAS PubMed.
- T. M. Klapötke, in High Energy Density Materials, ed. T. M. Klapötke, Springer, Berlin, Heidelberg, 2007, p. 85 Search PubMed.
- R. D. Chapman, in High Energy Density Materials, ed. T. M. Klapötke, Springer, Berlin, Heidelberg, 2007, p. 123 Search PubMed.
- G. Steinhauser, K. Tarantik and T. M. Klapötke, Copper in pyrotechnics, J. Pyrotech., 2008, 27, 3–13 CAS.
- M. Friedrich, J. C. Gálvez-Ruiz, T. M. Klapötke, P. Mayer, B. Weber and J. J. Weigand, BTA copper complexes, Inorg. Chem., 2005, 44, 8044–8052 CrossRef CAS PubMed.
- G. Steinhauser, K. Karaghiosoff and T. M. Klapötke, Synthesis and Crystal Structure of (CH3NH3)2[Cu(NO3)4]: a Rare Example of a Tetranitratocuprate(II) with a Light Cation of the Type M2[Cu(NO3)4], Z. Anorg. Allg. Chem., 2008, 634, 892–894 CrossRef CAS.
- B. C. Tappan, M. H. Huynh, M. A. Hiskey, D. E. Chavez, E. P. Luther, J. T. Mang and S. F. Son, Ultralow-density nanostructured metal foams: combustion synthesis, morphology, and composition, J. Am. Chem. Soc., 2006, 128, 6589–6594 CrossRef CAS PubMed.
- Q. Yang, S.-P. Chen, G. Xie and S.-L. Gao, Synthesis and characterization of an energetic compound Cu(Mtta)2(NO3)2 and effect on thermal decomposition of ammonium perchlorate, J. Hazard. Mater., 2011, 197, 199–203 CrossRef CAS PubMed.
- M. A. Petrie, J. A. Sheehy, J. A. Boatz, G. Rasul, G. K. Surya Prakash, G. A. Olah and K. O. Christe, Novel high-energy density materials. Synthesis and characterization of triazidocarbenium dinitramide, perchlorate, and tetrafluoroborate, J. Am. Chem. Soc., 1997, 119, 8802–8808 CrossRef CAS.
- Y.-C. Li, C. Qi, S.-H. Li, H.-J. Zhang, C.-H. Sun, Y.-Z. Yu and S.-P. Pang, 1,1′-Azobis-1,2,3-triazole: A high-nitrogen compound with stable N8 structure and photochromism, J. Am. Chem. Soc., 2010, 132, 12172–12173 CrossRef CAS PubMed.
- Y.-F. Zeng, X. Hu, F.-C. Liu and X.-H. Bu, Azido-mediated systems showing different magnetic behaviors, Chem. Soc. Rev., 2009, 38, 469–480 RSC.
- Y.-Q. Wang, Q. Yue, Y. Qi, K. Wang, Q. Sun and E.-Q. Gao, Manganese (II), Iron (II), and Mixed-Metal Metal–Organic Frameworks Based on Chains with Mixed Carboxylate and Azide Bridges: Magnetic Coupling and Slow Relaxation, Inorg. Chem., 2013, 52, 4259–4268 CrossRef CAS PubMed.
- S. S. Tandon, S. D. Bunge, J. Sanchiz and L. K. Thompson, Structures and magnetic properties of an antiferromagnetically coupled polymeric copper (II) complex and ferromagnetically coupled hexanuclear nickel (II) clusters, Inorg. Chem., 2012, 51, 3270–3282 CrossRef CAS PubMed.
- S. Biswas, S. Naiya, C. J. Gómez-García and A. Ghosh, Synthesis of the first heterometalic star-shaped oxido-bridged MnCu3 complex and its conversion into trinuclear species modulated by pseudohalides (N3−, NCS− and NCO−): Structural analyses and magnetic properties, Dalton Trans., 2012, 41, 462–473 RSC.
- H. Zhao, Z.-R. Qu, H.-Y. Ye and R.-G. Xiong, In situ hydrothermal synthesis of tetrazole coordination polymers with interesting physical properties, Chem. Soc. Rev., 2008, 37, 84–100 RSC.
- T. M. Klapötke and S. Jörg, Azidoformamidinium and 5-aminotetrazolium dinitramide—two highly energetic isomers with a balanced oxygen content, Dalton Trans., 2009, 4, 643–653 RSC.
- V. R. Pedireddi and S. Varughese, Solvent-dependent coordination polymers: Cobalt complexes of 3,5-dinitrobenzoic acid and 3,5-dinitro-4-methylbenzoic acid with 4,4′-bipyrdine, Inorg. Chem., 2004, 43, 450–457 CrossRef CAS PubMed.
- S. Varughese and V. R. Pedireddi, Hydrogen bond mediated open-frame networks in coordination polymers: supramolecular assemblies of Pr(III) and 3,5-dinitro-4-methylbenzoic acid with aza-donor compounds, Chem. Commun, 2005, 1824–1826 RSC.
- G. Singh and S. P. Felix, Studies on energetic compounds. Part 32: crystal structure, thermolysis and applications of NTO and its salts, J. Mol. Struct., 2003, 649, 71–83 CrossRef CAS.
- G. Morgant, J. d'Angelo, D. Desmaële, E. Dichi, M. Sghaier, B. Fraisse, P. Retailleau, V. Brumas, M. M. L. Fiallo and A. Tomas, Crystal structures of two (3-hydroxy-4-nitrobenzoato) complexes of magnesium(II): Ionic entities stabilized by stacking interactions and extensive hydrogen bonding, Polyhedron, 2008, 27, 2097–2104 CrossRef CAS.
- W.-H. Zhang and J.-S. Zhao, A uranium–potassium-organic framework solids: Hydrothermal synthesis, structure, and property of K[(UO2)3(μ3-OH)3(μ2-OH)(C7H4O4N)2]OH, Inorg. Chem. Commun., 2006, 9, 397–399 CrossRef CAS.
- Y. Qu and J.-J. Peng, Tetraamminebis(4-nitrobenzoato-κO)copper(II), Acta Crystallogr., Sect. E: Struct. Rep. Online, 2006, 62, m952–m953 CAS.
- Explosives, ed. R. Meyer and J. Kohler, Revised and extended, VCH Publishers, New York, 1993, vol. 149 Search PubMed.
- Explosives, ed. R. Meyer and J. Kohler, Revised and extended, VCH Publishers, New York, 4th edn, 1993, vol. 197 Search PubMed.
- G. M. Sheldrick, SHELXS-97, Program for X-ray Crystal Structure Determination, University of Göttingen, Germany, 1997 Search PubMed.
- G. M. Sheldrick, SHELXL-97, Program for X-ray Crystal Structure Refinement, University of Göttingen, Germany, 1997 Search PubMed.
- R. Meyer, J. Köhler and A. Homburg, Explosives, Wiley–VCH Verlag GmbH & Co. KGaA, Weinheim, 2007 Search PubMed.
- J. Koehler and R. Meyer, Explosivstoffe, Wiley-VCH, Weinheim, Germany, 9th edn, 1998 Search PubMed.
- T. M. Klapötke and C. M. Sabaté, Bistetrazoles: nitrogen-rich, high-performing, insensitive energetic compounds, Chem. Mater., 2008, 20, 3629–3637 CrossRef.
- M. Anniyappan, M. B. Talawar, G. M. Gore, S. Venugopalan and B. R. Gandhe, Synthesis, characterization and thermolysis of 1,1-diamino-2,2-dinitroethylene (FOX-7) and its salts, J. Hazard. Mater., 2006, 137, 812–819 CrossRef CAS PubMed.
- H. E. Kissinger, Reaction kinetics in differential thermal analysis, Anal. Chem., 1957, 29, 1702–1706 CrossRef CAS.
- T. Ozawa, A new method of analyzing thermogravimetric data, Bull. Chem. Soc. Jpn., 1965, 38, 1881–1886 CrossRef CAS.
- C. D. Doyle, Kinetic analysis of thermogravimetric data, J. Appl. Polym. Sci., 1961, 5, 285–292 CrossRef CAS.
- R.-Z. Hu, Z.-Q. Yang and Y.-J. Liang, The determination of the most probable mechanism function and three kinetic parameters of exothermic decomposition reaction of energetic materials, Thermochim. Acta, 1988, 123, 135–151 CrossRef.
- Y.-X. Ou, Explosives, Beijing Institute of Technology Press, China, 2006 Search PubMed.
- J. D. Cox, D. D. Wagman and V. A. Medvedev, CODATA Key Values for Thermodynamics, Hemisphere Publishing Corp, New York, 1989 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The CIF file gives crystallographic data for the complex. For Fig. S1 and Table S1 see ESI. CCDC 970127. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra00635f |
| This journal is © The Royal Society of Chemistry 2014 |