Universal solution-processed high-k amorphous oxide dielectrics for high-performance organic thin film transistors
Xurong Zhaoa,
Sumei Wang*a,
Aiju Lia,
Jun Ouyang*a,
Guodong Xia*b and
Ji Zhouc
aKey Laboratory for Liquid-Solid Structural Evolution and Processing of Materials (Ministry of Education), Shandong University, Jinan 250061, China. E-mail: wangsumei3000@sdu.edu.cn; ouyangjun@sdu.edu.cn
bDepartment of Material and Engineering, Qilu University of Technology, Jinan 250353, China. E-mail: gdxia@live.cn
cState Key Laboratory of New Ceramic and Fine Processing, Tsinghua University, Beijing, 100084, China
First published on 11th March 2014
Abstract
Solution-processed high-k dielectrics for organic thin film transistors (OTFTs) have been widely studied with the objective of achieving high-performance and low cost OTFTs for next-generation electronics. In this study, we introduce a universal solution fabrication of high-k amorphous oxide dielectrics for high-performance OTFTs. The amorphous oxide films by solution processes feature a smooth surface, uniform composition and excellent dielectric properties. With ZrTiOx as a typical example, a k value and capacitance as high as 53 and 467 nF cm−2 could be achieved. The polystyrene (PS) modification of ZrTiOx dielectric films results in a leakage current as low as 4 × 10−8 A cm−2. Based on their implementation as a gate insulator, the solution-processed high-k ZrTiOx dielectric films realize high and stable performance OTFTs during operation at a low-voltage. A carrier mobility of 0.58 cm2 V−1 s−1, an on/off current ratio of 104, and a low operating voltage of 6 V were achieved through simple control of the annealing temperature. Our results show the possibility of the solution-processed high-k amorphous oxide dielectric layer as a gate insulator for OTFTs. We believe that these high-k amorphous oxide dielectric films offer great potential for next-generation high-performance organic electronics, especially low-voltage electronics.
Introduction
Organic thin film transistors (OTFTs) have attracted considerable interest for low cost electronic devices such as radio-frequency identification (RFID) tags, sensors, and electronic paper.1–4 For organic semiconductors, the intrinsically low carrier motilities could limit the performance of OTFTs. To achieve desirable channel currents, relatively high operating voltages (>20 V) are mostly required, which may lead to high power consumption in organic electronics. To overcome this key drawback, a feasible solution is to use a high dielectric constant (k) layer in OTFT devices.5 In the last years, various high-k oxide dielectrics, such as Al2O3,6 HfO2,7 ZrO2,8 Y2O3 (ref. 9) and La2O3,10 have attracted extensive research efforts for low-voltage organic devices. To obtain a high-k value, the highly crystalline oxides are usually indispensable. Nevertheless, the grain boundary in polycrystalline films could become the channel of leakage current, limiting the performance of TFTs. Moreover, the mechanical stability, films smoothness and device uniformity of polycrystalline thin films are less desired for high-performance electronic devices. In contrast, recent results suggested that the corresponding amorphous oxides with smooth surface and high density offer intriguing and potentially useful properties for the high-performance TFTs.11–13 In particular, complex oxides, such as alloys of Zr, Hf and La oxides, are often used to have better dielectric properties.14–16 However, the low k (<10) values of most amorphous oxides greatly limit the large-scale fabrication of high-performance OTFTs.In the last years, various vapor routes, such as chemical vapor deposition,17 atomic layer deposition,18 sputtering,19 and pulsed laser deposition,20 are usually used for the preparation of oxide dielectric layers. For example, ZrxTiyOz thin films by atomic layer deposition possess the high-k of 45–65, and the leakage current of 10−4 A cm−2 at 0.2 MV cm−1.21 However, these vapor growth techniques are mostly high cost due to the high vacuum equipment, leading to limited production and throughout. In contrast, solution methods, like sol–gel process, have attracted intensive interests due to the inexpensive equipment, large-scale production, and high throughput deposition. Recently, ultraviolent (UV) irradiation of sol–gel films have been shown as an effective method for the room temperature fabrication of high-k oxide dielectric films.22,23 Amorphous ZrTiOx films can achieve a high-k of 18.9.23 Nevertheless, the high cost of short wavelength UV equipment may limit the mass production. Therefore, it is of great significance to facilely and low-lost fabricate high-k amorphous oxide dielectrics for high-performance electronics.
In this paper, we report a general strategy on the scalable fabrication of high-k amorphous complex oxide dielectric films ZrTiOx by a simple sol–gel spin-coating method. We found the suppressed crystallization and significantly high density of complex oxides lead to high dielectric properties, such as a high dielectric constant of 53 and high capacitance of 467 nF cm−2. As a proof of concept, a high-performance low-voltage pentacene-based OTFT with a low operating voltage of 6 V, high carrier mobility of 0.58 cm2 V−1 s−1, and on/off current ratio of 104, was realized with the polystyrene (PS) modified high-k ZrTiOx dielectric layer. Consequently, the solution-processed high-k amorphous oxide dielectric films provide an effective approach for high-performance organic electronics, especially low-voltage transistors and memories.
Experimental
Preparation of amorphous oxide dielectric films
In a typical process for ZrTiOx film, 0.4 mmol zirconium oxychloride octahydrate (ZrOCl2·8H2O) was dissolved in 1 ml 2-methoxyethanol (HOCH2CH2OCH3) to form zirconium precursor solution, while 0.4 mmol tetrabutyl titanate (Cl6H36O4Ti) stabilized by acetylacetone was dissolved in 1 ml 2-methoxyethanol to form titanium precursor solution. Then the ZrTiOx precursor solution was obtained by thoroughly mixing the two solutions and stirring at 100 °C for 60 min to obtain a transparent precursor solution. The precursor solution was spin-coated on p-type silicon substrate at 2500 rpm for 60 s to form a uniform ZrTiOx film at room temperature. Then the sample was preheated at 300 °C for 10 min to remove the volatile organic components. These processes were repeated several times to obtain desired thickness. The as-deposited ZrTiOx films were then annealed at 400 to 750 °C in an air furnace for 2 hours. For comparison, the pure ZrOx films were also prepared with the same process. The preparation of LaTiOx film is similar to that of ZrTiOx film by just changing the metal precursor (La(NO3)3 was selected as precursor).Fabrication of OTFTs
To fabricate the pentacene-based OTFTs, a heavily doped n-type Si (100) wafer was used as the substrate. All of the substrates were carefully cleaned in acetone, followed with ethanol and deionized water using ultrasonication for 10 minutes each time. After that, the substrates were spin-coated with ZrTiOx precursor solution and annealed at setting temperature to obtain a smooth and dense layer. To improve the interface compatibility of amorphous oxide and organic active layers, a thin polystyrene (PS) layer was prepared on the ZrTiOx films by a simple spin-coating process. 0.1 g PS was dissolved in 1 ml toluene to form a transparent solution. Then the PS solution was spin-coated on the ZrTiOx films at 3000 rpm for 60 s and vacuum dried at 120 °C for 60 min to form PS layer. After that, the pentacene active layer and silver source–drain were deposited by thermal evaporation process. In a typical process, 50 nm thick pentacene was evaporated onto the PS–ZrTiOx dielectrics at a rate of 0.2 Å s−1 in a base pressure of 2.5 × 10−4 Pa. Then 80 nm silver source and drain electrodes were evaporated onto the pentacene through a shadow mask. The thickness of pentacene and silver layer were controlled by a calibrated quartz crystal monitor. The channel length (L) and the width (W) were fixed at 100 and 2000 μm, respectively.Characterizations
The crystalline and phase were examined by Rigaku D/max-2500X-ray diffraction (XRD) using Cu Kα radiation analysis. Hitachi S-4800 scanning electron microscopy (SEM) and Veeco Dimension 3100 atomic force microscope (AFM) system were used to study the microstructure and surface. For leakage and capacitance characterization, a metal–insulator–metal (MIM) structure was fabricated by depositing 50 nm thick gold dots with diameter of 200 μm onto the dielectric layer through a shadow mask. The frequency dependence of capacitance density was tested by 7600 Plus Precision LCR Meter at a frequency of 100 Hz–100 KHz. The leakage properties were measured by Keithley 2400 Source-Meter. OTFTs were characterized using two Keithley 2400 Source-Meter units. All characterizations were carried out at room temperature in ambient air.Results and discussion
Microstructure of amorphous oxide dielectric films
The crystalline and phase condition of ZrTiOx films annealed at temperatures from 600 to 750 °C were first studied, and the X-ray diffraction pattern (XRD) patterns are shown in Fig. 1a. The results revealed that the ZrTiOx films are amorphous when annealed below 600 °C, and begin to crystallize at about 650 °C. When the annealed temperature increased to 750 °C, the strong diffraction peaks are indexed to single ZrTiO4 phase with high crystalline state. For comparison, the XRD patterns of the pure ZrO2 film by sol–gel process are shown in Fig. 1b. It can be seen that the pure ZrO2 films remained amorphous state annealed at 400 °C, and crystallize at 450 °C. These results clearly suggested that the crystallization onset temperature of the ZrTiOx complex oxide films was delayed at least 200 °C with the incorporation of TiO2. | ||
| Fig. 1 XRD patterns of (a) ZrTiOx, (b) ZrOx (c) LaTiOx, and (d) La2O3 thin films at different annealing temperatures. | ||
To demonstrate the universality of our strategy, we also prepared LaTiOx films with the addition of TiO2. The XRD patterns in Fig. 1c revealed the single La2Ti2O7 phase formed with the crystallization temperature about 800 °C. In contrast, La2O3 films with minor La2CO5 begin to crystallize between 500 to 600 °C, as shown in Fig. 1d. Therefore, the introduction of TiO2 offers an effective route to delay the crystallization onset temperature of metal oxides. For oxide dielectrics used in OTFTs, amorphous oxide dielectrics are expected to avoid the usually large leakage current caused by the grain boundary of polycrystalline oxide, benefiting the high-performance OTFT devices. The suppressed crystallization of oxide provided us novel routes to amorphous materials with potentially higher density, important for high-k dielectric materials.
The amorphous state of ZrTiOx films is proved by the microstructure characterization. Fig. 2a–c displays scanning electron micrograph (SEM) images of amorphous ZrTiOx films deposited on Si substrates. The surface of ZrTiOx films is quite smooth due to the amorphous state, regardless of different annealing temperatures of 400–600 °C. The cross-section SEM image in the inset of Fig. 2b shows the thickness of ZrTiOx film annealed at 500 °C is about 100 nm. Fig. 2d–f shows the tapping mode atomic force micrograph (AFM) images of sol–gel ZrTiOx films annealed at 400–600 °C. The scanning area is 3 μm × 3 μm. As can been seen, all the ZrTiOx films exhibits a uniform and smooth surface without any surface defects and pinholes. The film root mean square (RMS) roughness decreased with the increase of annealing temperature. The RMS values of ZrTiOx films annealed at 400–600 °C are 0.34, 0.26, and 0.25 nm, respectively. These small RMS values are comparable to that of thermal SiO2 (RMS ∼ 0.19 nm). The smooth surface and high quality ZrTiOx are beneficial for the charge carrier transport of organic semiconductors, since rough dielectric surface could induce the physical traps, or disturb the growth of organic semiconductors.23
Dielectric properties of amorphous oxide dielectric films
To prove the high dielectric properties of the complex oxide films with the addition of TiO2, we measured the capacitance for the ZrTiOx and LaTiOx film, as shown in Fig. 3. The thickness of all the films annealed at 500 °C was controlled to be 100 nm. As shown in Fig. 3a, the capacitance of pure ZrO2 films is about 230 nF cm−2 at the frequency range from 100 Hz to 100 KHz. With the addition of TiO2, the capacitance of ZrTiOx increases dramatically, and the capacitance is as high as 560 nF cm−2 at the low frequency of 100 Hz. At the high frequency, the capacitance decreases to about 350 nF cm−2. This large dielectric dispersion of the ZrTiOx film at low frequency is similar to the previous results.24,25 At present the exact explanation for this large dielectric dispersion is not clear. It may be related to the charged structural defects, such as the oxygen vacancy V˙˙o, which forman electric dipole and lead to the dielectric dispersion.Since OTFTs are normally worked in low frequency circuits, this high capacitance value of the ZrTiOx dielectrics obtained at low frequency can meet the requirement of most OTFT applications.26 For the La2O3 films, the addition of TiO2 can also increase the capacitance from 148 to 162 nF cm−2 at 100 Hz, as shown in Fig. 3b. Among the various high-k oxides, titanium dioxide (TiO2) is promising because of its high dielectric constant of approximately 80. However, its poor conduction band offsets with Si makes it difficult to act as an effective dielectric material. The improved capacitance for the ZrTiOx and LaTiOx films indicated that the complex oxide by the addition of TiO2 is an effective strategy for the enhancement of dielectric properties.21,23
In the following part, we will focus on the ZrTiOx films to show high-performance amorphous oxide dielectric films for organic electronic applications. The dielectric properties of ZrTiOx films annealed at different temperatures were characterized by capacitance–frequency (C–f) curve, as shown in Fig. 4a. The capacitance density of the ZrTiOx films decreases with the increasing frequency. At 1 KHz, the capacitance density of the ZrTiOx films annealed at 500 °C is about 380 nF cm−2 while those annealed at 400 and 600 °C are 443 and 467 nF cm−2, respectively. The effective k value is calculated by the following formula:
 | (1) |
 | ||
| Fig. 4 Dielectric characteristics (a) capacitance property and (b) leakage current of ZrTiOx thin films at different annealing temperatures. | ||
To improve the interface compatibility of amorphous oxide and organic active layers, ZrTiOx films were modified with 10 nm PS layer. Due to the low permittivity of PS layer (k ∼ 2.5) and the increased thickness of ZrTiOx–PS dielectric layer, the equivalent capacitance of the hybrid dielectrics layer decreases to 158, 137 and 168 nF cm−2 at 1 KHz for ZrTiOx films annealed at 400, 500 and 600 °C, respectively, as shown in Fig. 4a. Although the total capacitance of PS–ZrTiOx hybrid dielectric films decrease, the remarkably decreased leakage current density was achieved. As shown in Fig. 4b, the leakage current is about 5 × 10−6 A cm−2 at −4 V for the ZrTiOx films annealed at 400 °C, while it increased to be over 10−5 A cm−2 for the ZrTiOx films annealed at 500 and 600 °C. In contrast, with the modification of 10 nm PS layer, the leakage current of the 500 °C annealed ZrTiOx decreased to 4 × 10−8 A cm−2, as shown in Fig. 4b. The significantly decreased leakage current by the PS layer is highly desirable for high-performance OTFT devices.
OTFT applications of amorphous oxide dielectric films
To demonstrate the effectiveness of our high-k amorphous complex oxide dielectrics, bottom-gate, top-contact pentacene-based OTFTs devices with silver source–drain electrode were fabricated with PS modified ZrTiOx dielectric films. The schematic structure is shown in the inset part of Fig. 5a. All the device measurements were performed in ambient air. The output and transfer characteristics curves of OTFTs are shown in Fig. 5. The VG varies from 0 to −6 V with a step of 1 V. All the devices have typical p-type OTFT characteristics with a linear region at small source–drain voltages (VDS) and an excellent saturation region when the VDS values near the gate voltages. In transfer characteristic curves, the VDS was kept at −6 V, the field effect mobility (μ) in the saturation region is obtained by the following equation:
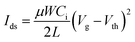 | (2) |
| Temperature (°C) | Ci (nF cm−2) | k | Mobility (cm2 V−1 s−1) | Ion/off ( × 104) | Vth (V) |
|---|---|---|---|---|---|
| 400 | 443 | 50 ± 0.5 | 0.35 ± 0.02 | 2.90 | −2.27 |
| 500 | 380 | 42 ± 0.3 | 0.47 ± 0.03 | 5.57 | −2.50 |
| 600 | 467 | 53 ± 0.5 | 0.58 ± 0.03 | 1.99 | −2.54 |
The improved mobility of ZrTiOx dielectric layers with the annealing temperature can be attributed to the synthetic effect of the decreased surface roughness and the decreased traps densities. First, with the increase of the annealing temperature, the surface roughness of the ZrTiOx dielectric layer decreases from 0.34 to 0.25 nm, as shown in Fig. 2. The decreased surface roughness of the dielectric layer leads to the increased grain size of the semiconductor, and the ordered growth of semiconductor.27 The morphology and crystallite of the pentacene deposited onto the PS–ZrTiOx dielectric layer were investigated. As shown in Fig. 6, all these pentacene films exhibit a dendritic structure with well-formed terraces. For ZrTiOx dielectrics annealed at 400 °C, some small and round grain dispersed between large pentacene grains, and the average grain size of pentacene is about 1.2 μm. When the annealing temperature increased to 500 and 600 °C, the average grain size of pentacene increased to 1.5 μm, with less amount of small grains marked with the red circles in Fig. 6. As a result, the enhanced order and the reduced grain-boundary-induced scattering of pentacene will benefit the charge transport between the source and drain, and improve the performance of OTFTs. Moreover, the higher annealing temperature leads to a more dense structure and less defects of ZrTiOx dielectric layer, enhancing the charge carrier transport.28
 | ||
| Fig. 6 AFM Surface morphology of the pentacene film deposited on the hybrid PS–ZrTiOx dielectric films at different annealing temperatures of (a) 400, (b) 500, and (c) 600 °C. | ||
Conclusions
In summary, a universal solution fabrication of high-k amorphous oxide dielectric layers was demonstrated for high-performance OTFTs. A high dielectric constant of 53 and high capacitance of 467 nF cm−2 were achieved for the ZrTiOx films through simple control of annealing temperature. The interface modification of amorphous oxide dielectric films with polymer layer lead to the leakage current as low as 4 × 10−8 A cm−2. As a result, the polymer modified high-k amorphous oxide dielectric films realize high-performance OTFTs, such as a high carrier mobility of 0.58 cm2 V−1 s−1, on/off current ratio of 104 and low operating voltage of 6 V. Our simple solution preparation of high-k amorphous oxide dielectric films could provides novel routes for high-performance organic electronics, especially low-voltage organic transistor and memory devices.Acknowledgements
This work was supported by the National Natural Science Fountain of China (no. 61106086), the Promotive research fund for excellent young and middle-aged scientists of Shandong Province (BS2011DX014), and opening foundation of State Key Laboratory of New Ceramic and Fine Processing in Tsinghua University (no. KF201311).Notes and references
- Y. G. Wen, Y. Q. Liu, Y. L. Guo, G. Yu and W. P. Hu, Chem. Rev., 2011, 111, 3358 CrossRef CAS PubMed.
- M. A. McCarthy, B. Liu, E. P. Donoghue, I. Kravchenko, D. Y. Kim, F. So and A. G. Rinzler, Science, 2011, 332, 570 CrossRef CAS PubMed.
- C. Wang, H. Dong, W. Hu, Y. Liu and D. Zhu, Chem. Rev., 2011, 112, 2208 CrossRef PubMed.
- C. Liao and F. Yan, Polym. Rev., 2013, 53, 352 CrossRef CAS.
- R. P. Ortiz, A. Facchetti and T. J. Marks, Chem. Rev., 2009, 110, 205 CrossRef PubMed.
- S. H. Chae, W. J. Yu, J. J. Bae, D. L. Duong, D. Perello, H. Y. Jeong, Q. H. Ta, T. H. Ly, Q. A. Vu, M. Yun, X. Duan and Y. H. Lee, Nat. Mater., 2013, 12, 403 CrossRef CAS PubMed.
- J. W. Liu, M. Y. Liao, M. Imura and Y. Koide, Appl. Phys. Lett., 2013, 103, 092905 CrossRef.
- J. Jang, R. Kitsomboonloha, S. L. Swisher, E. S. Park, H. Kang and V. Subramanian, Adv. Mater., 2013, 25, 1042 CrossRef CAS PubMed.
- H. H. Hsu, C. Y. Chang and C. H. Cheng, Appl. Phys. A, 2013, 112, 817 CrossRef CAS.
- R. Sarma, D. Saikia, K. Konwar and B. Baishya, Indian J. Phys., 2009, 84, 547 CrossRef.
- C. Avis, Y. G. Kim and J. Jang, J. Mater. Chem., 2012, 22, 17415 RSC.
- O. Acton, I. Osaka, G. Ting, D. Hutchins, H. Ma, R. D. McCullough and A. K. Y. Jen, Appl. Phys. Lett., 2009, 95, 113305 CrossRef.
- Q. Wei, E. You, N. R. Hendricks, A. L. Briseno and J. J. Watkins, ACS Appl. Mater. Interfaces, 2012, 4, 2322 CAS.
- J. Ko, J. Kim, S. Y. Park, E. Lee, K. Kim, K.-H. Lim and Y. S. Kim, J. Mater. Chem. C, 2014, 2, 1050 RSC.
- G. He, Z. Sun, G. Li and L. Zhang, Crit. Rev. Solid State Mater. Sci., 2012, 37, 131 CrossRef CAS.
- M. Suzuki, Materials, 2012, 5, 443 CrossRef CAS.
- A. J. Ben-Sasson, G. Ankonina, M. Greenman, M. T. Grimes and N. Tessler, ACS Appl. Mater. Interfaces, 2013, 5, 2462 CAS.
- T. Shinya, L. Zhongda and T. P. Chow, Jpn. J. Appl. Phys., 2013, 52, 08JN24 CrossRef.
- Y. Lu, C. Le Paven, H. V. Nguyen, R. Benzerga, L. Le Gendre, S. Rioual, F. Tessier, F. Cheviré, A. Sharaiha, C. Delaveaud and X. Castel, Cryst. Growth Des., 2013, 13, 4852 CAS.
- G. Giusti, J. Bowen, Q. Ramasse, G. Rey, E. Blackburn, L. Tian, I. P. Jones and J. S. Abell, Thin Solid Films, 2012, 524, 249 CrossRef CAS.
- A. Rahtu, M. Ritala and M. Leskelä, Chem. Mater., 2001, 13, 1528 CrossRef CAS.
- Y. M. Park, J. Daniel, M. Heeney and A. Salleo, Adv. Mater., 2011, 23, 971 CrossRef CAS PubMed.
- G. Xia, S. Wang, X. Zhao and L. Zhou, J. Mater. Chem. C, 2013, 1, 3291 RSC.
- P. Victor, S. Bhattacharyya and S. B. Krupanidhi, J. Appl. Phys., 2003, 94, 5135–5142 CrossRef CAS.
- K. Morii, H. Kawano, I. Fujii, T. Matsui and Y. Nakayama, J. Appl. Phys., 1995, 78, 1914–1919 CrossRef CAS.
- J. Li, Z. Sun and F. Yan, Adv. Mater., 2012, 24, 88 CrossRef CAS PubMed.
- X. Sun, C.-a. Di and Y. Liu, J. Mater. Chem., 2010, 20, 2599 RSC.
- K. H. Cheng, W. M. Tang, L. F. Deng, C. H. Leung, P. T. Lai and C. M. Che, J. Appl. Phys., 2008, 104, 116107 CrossRef.
| This journal is © The Royal Society of Chemistry 2014 |



