Two-dimensional assemblies of ultrathin titanate nanosheets for lithium ion battery anodes†
Abstract
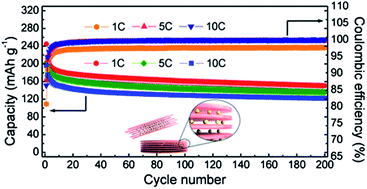
Ultrathin titanate nanosheets of 0.5 nm thickness were successfully synthesized from the non-hydrolytic sol–gel reaction of tetraoctadecyl orthotitanate via a heat-up method. The synthesized nanosheets were easily assembled to be layer structures by being reacted with hydroxide ions in basic solutions such as LiOH, NaOH, and KOH. The hydrophobic surface of the titanate nanosheets was also modified to be a hydrophilic surface through an assembly process. The layer structured nanosheets were employed as anode materials for lithium ion batteries to visualize fast charging and discharging effects utilizing 2D structured electrodes, and stable cycling induced the mechanically stable layered structure. The ultrathin morphology of the 2D titanate electrodes affected not only the diffusion path of Li ions but also the reaction mechanism from the insertion reaction of the crystal interior to the surface reaction. Furthermore, the electrodes of the layer structured nanosheets had superior cycling and rate performances.

 Please wait while we load your content...
Please wait while we load your content...