Selective colorimetric and ratiometric probe for Ni(II) in quinoxaline matrix with the single crystal X-ray structure†
Shyamaprosad Goswami*a,
Shampa Chakrabortya,
Avijit Kumar Dasa,
Abhishek Mannaa,
Aditya Bhattacharyyab,
Ching Kheng Quahc and
Hoong-Kun Funcd
aDepartment of Chemistry, Bengal Engineering and Science University, Shibpur, Howrah 711103, West Bengal, India. E-mail: spgoswamical@yahoo.com; Fax: +91-3326682916
bDepartment of Chemistry, Indian Institute of Technology, Kanpur 208016, India
cX-ray Crystallography Unit, School of Physics, Universiti Sains Malaysia, 11800 USM, Penang, Malaysia. E-mail: ckquah@usm.my; hkfun@usm.my
dDepartment of Pharmaceutical Chemistry, College of Pharmacy, King Saud University, P.O. Box 2457, Riyadh 11451, Saudi Arabia. E-mail: hfun.c@ksu.edu.sa
First published on 20th March 2014
Abstract
A quinoxaline based colorimetric nickel sensor, HQAP [2-(quinoxalin-2-ylmethyleneamine)phenol] with high selectivity and sensitivity toward Ni2+ ions is shown to have potential for practical use. The absorption maximum of HQAP shows a large ratiometric shift from 306 to 570 nm (ΔI = 264 nm) in the presence of Ni2+ ions, and the color changes from colorless to deep violet only upon addition of Ni2+ which is very easily observed by the naked eye (the detection limit of Ni2+ is as low as 4.16 μM in solution). The predicted binding mode (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) from spectral analysis and Job's plot was confirmed by the single crystal X-ray structure of the complex.
1) from spectral analysis and Job's plot was confirmed by the single crystal X-ray structure of the complex.
Nickel is widely used in various industrial applications such as in Ni–Cd batteries, electroplating, rods for arc welding, pigments for paints, ceramics, surgical and dental prostheses, catalysts for hydrogenation and as magnetic tapes for computers. Enzymes of some microorganisms and plants contain nickel as an active site, which makes the metal an essential nutrient for them. On the other hand, it is also a toxic metal and known to cause pneumonitis, asthma and cancer of the lungs and also cause disorders of the respiratory and central nervous systems.1–6 A number of methods, such as atomic absorption spectrometry (AAS), flame atomic absorption spectrometry-electro thermal atomization (AAS-ETA),7,8 ICP-AES and flame photometry9 can be used for the determination of nickel. These methods provide accurate results but are not very appropriate for the analysis of a large number of environmental samples because they require appropriate expertise and good infrastructure. On the other hand, selective metal ion sensors are very useful for the monitoring of heavy metals in a large number of samples as they are cheaper, convenient and easy to operate and generally they require no sample pre-treatment. There is a requirement to impart selectivity to the ion sensor for a material that the material has a strong affinity for a particular metal ion but has poor sensitivity to others. However in reality such good sensors for nickel are very rare, particularly in a quinoxaline matrix.10,11
Ratiometric colorimetric probes can enable the measurement of absorption intensities at two different wavelengths, providing a built-in correction for environmental effects and increasing the dynamic range of absorption measurement. This was considered to be a good approach to overcome the major limitation of intensity based probes, for which variations in the environmental sample and probe distribution were problematic for quantitative measurements. However, so far, ratiometric and colorimetric probes for Ni(II) are still very rare.12–18
We report here a remarkably simple but very efficient chemosensor HQAP19 for nickel by the simple condensation reaction between quinoxaline aldehyde20 and 2-aminophenol in dry methanol at room temperature (Scheme 1). Here, we use quinoxaline as the key part of the receptor as the electron withdrawing part of HQAP unit and 2-aminophenol due to its electron-donating hydroxyl group which provides a suitable cavity for the binding of metal ions. The sensing properties of the receptor were investigated by monitoring the UV-vis absorption spectral behavior upon addition of various metal ions such as Na+, K+, Fe3+, Cu2+, Mn2+, Ag+, Ca2+, Zn2+, Hg2+, Cr3+, Mg2+, Pb2+ and Ni2+ ions.
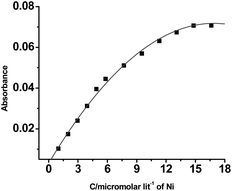
The spectroscopic studies of HQAP (1 × 10−5 M) were carried out in acetonitrile by using different interfering metal ions having 2 × 10−4 M strength. As shown in Fig. 1, the UV-vis spectra of the receptor (HQAP) are characterized by the two characteristic bands centered at 341 and 372 nm. Upon gradually increasing the nickel ion concentration, the bands at 341 and 372 nm gradually disappear and a new band appears at 570 nm with an isosbestic point at 432 nm, indicating the formation of a complex20 between the receptor and nickel (Fig. 1) which is also responsible for the generation of a deep violet color after the addition of nickel chloride into the solution of the receptor. Fig. 2 actually indicates the change of absorbance with the concentration of nickel. From the UV-vis titration data, it is revealed that a minimum 4.16 μM of Ni2+ can be detected by using 10 μM of receptor HQAP using the equation DL = K × Sb1/S, where K = 3, Sb1 is the standard deviation of the blank solution and S is the slope of the calibration curve (ESI†).
After addition of 1.6 equivalents of nickel chloride, it reaches a saturation level. Titrations were also carried out with various cations, such as Na+, K+, Fe3+, Cu2+, Mn2+, Ag+, Ca2+, Zn2+, Hg2+, Cr3+, Mg2+, Pb2+, Pt2+, Pd2+ and Co2+ as their chloride salts. Interestingly, there is no obvious change observed in the UV-vis spectra except with Co2+ which shows a slight interference (ESI†). The slight appearance of a new peak at 557 nm indicates that the receptor (HQAP) has a slight response to cobalt and zinc ions due to their similar size and charge. The cavity of HQAP binds selectively to Ni2+ over Co2+ probably because the size of the cation perfectly fits.
Fig. 3 actually shows the selectivity for nickel over other cations which is shown by the sky blue bar. The slight interference of cobalt is shown by the grey bar but the Co2+ ion is not clearly detectable by the naked eye, as shown in Fig. 4. From the experimental data, it can be concluded that the receptor HQAP possesses high selectivity and sensitivity towards nickel in acetonitrile medium as well as in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 acetonitrile–HEPES buffer medium (ESI†). The other cations except cobalt and zinc had practically no significant influence. The color changes are most probably due to the formation of coordination bonds or deprotonation of –OH groups of receptor HQAP on the addition of nickel ions which is shown in Scheme 2.
1 acetonitrile–HEPES buffer medium (ESI†). The other cations except cobalt and zinc had practically no significant influence. The color changes are most probably due to the formation of coordination bonds or deprotonation of –OH groups of receptor HQAP on the addition of nickel ions which is shown in Scheme 2.
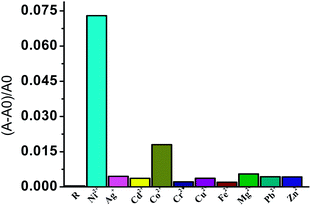 | ||
| Fig. 3 (A − A0)/A0 ratios of receptor HQAP (1 × 10−5 M) after the addition of 1.6 equivalents of each of the various cations of concentration 2 × 10−4 M in acetonitrile. | ||
 | ||
| Fig. 4 Color changes of receptor HQAP (1 × 10−5 M) upon addition of 0.8 equivalents of each of the different guest cations (2 × 10−4 M). | ||
These results suggest that HQAP could be exploited as a colorimetric “naked eye” sensor for Ni2+ ions amongst the various typical transition-metal ions such as Cu2+, Pb2+, and Hg2+ which are normally difficult to differentiate.
These coordination bonds or deprotonations affect the electronic properties of the chromophore which results in the change of color from colorless to deep violet, along with a new charge-transfer interaction between the nickel bound –OH moiety and the electron deficient quinoxaline moiety. A well-defined isosbestic point at 432 nm emerged during the spectral titrations, which indicated the formation of the stable complex with a certain stoichiometric ratio between the host and guest via internal charge transfer (ICT) with a new band which appeared at 570 nm. The 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry for the host–guest complexation was elaborated by the profile of the intensities of the decreasing band centered at 372 nm and increasing band at 570 nm which was also confirmed by the Job's plot analysis (Fig. 5). The crystal structure of the nickel(II) complex was determined by single crystal X-ray diffraction and the crystallographic data has been deposited with the Cambridge Crystallographic Data Center no. CCDC 964936.
1 stoichiometry for the host–guest complexation was elaborated by the profile of the intensities of the decreasing band centered at 372 nm and increasing band at 570 nm which was also confirmed by the Job's plot analysis (Fig. 5). The crystal structure of the nickel(II) complex was determined by single crystal X-ray diffraction and the crystallographic data has been deposited with the Cambridge Crystallographic Data Center no. CCDC 964936.
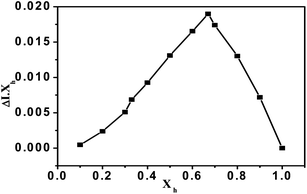 | ||
| Fig. 5 Job's plot diagram of receptor HQAP for Ni2+ cations (where Xh is the mole fraction of the host and ΔI indicates the change in the absorbance). | ||
Yusuff et al. reported the synthesis of several metal complexes20 of HQAP by refluxing in ethanol with metal diacetate (or chloride for Fe3+) on more than a gram scale. However in our UV-vis titration experiments, when the concentration of HQAP was 10−5 M we observed the sensing selectivity of Ni2+ with some interference from Co2+ and Zn2+ (less than Co2+) but no interference from other metal ions at that concentration.
The first single crystal X-ray structural proof of the nickel–HQAP is demonstrated which shows the participation of the quinoxaline nitrogen in favor of the six membered ring along with the five membered one formed from the imino phenol moiety previously20 suggested by other spectral studies. The single crystal consists of a nickel(II) complex and a chloroform molecule in the asymmetric unit (Scheme 2). The two quinoxaline ligands exist in trans conformations with respect to the N1 = C7 and N4 = C22 bonds [1.278(7) and 1.292(8) Å]. The nickel(II) atom displays a distorted octahedral coordination geometry, provided by two N atoms [Ni–N = 2.025(5)–2.219(5) Å] and one O atom [Ni–O = 2.047(4) and 2.055(4) Å] of each quinoxaline ligand.
The binding constant of HQAP with nickel is found to be 1.26 × 105 M−1 from a non-linear least squares fit analysis method at 372 nm (ESI†). To further explore the binding mechanism, the Job's plot of the UV-vis titrations of Ni2+ ion with a total volume of 2 ml was obtained. A maximum absorption was observed when the molar fraction reached 0.67, which is indicative of a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complexation between HQAP and Ni2+ ions. The ESI mass spectrum of a mixture of HQAP and NiCl2·7H2O also revealed the formation of a 2
1 stoichiometric complexation between HQAP and Ni2+ ions. The ESI mass spectrum of a mixture of HQAP and NiCl2·7H2O also revealed the formation of a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand–metal complex through metal coordination interactions, with a major signal at m/z = 555.0 [for (2 M + Ni)+ ions]. From the IR data, the phenomenon is also well explained by the decreasing broadness of the –OH peak at 3372 cm−1 due to the insertion of nickel metal in HQAP (ESI†).
1 ligand–metal complex through metal coordination interactions, with a major signal at m/z = 555.0 [for (2 M + Ni)+ ions]. From the IR data, the phenomenon is also well explained by the decreasing broadness of the –OH peak at 3372 cm−1 due to the insertion of nickel metal in HQAP (ESI†).
Furthermore, to examine the selectivity of the probe in a complex background of potentially competing species, the absorbance of HQAP with Ni2+ was investigated in the presence of other metal ions. The experiment is performed by adding the species under investigation i.e. Ni2+ (2.0 equivalents) to the sensor in the presence of commonly employed interfering species i.e. metal ions (6.0 equivalents). With the exception of Co2+ and Zn2+ a background of competing metal ions did not interfere in the detection of Ni2+ by HQAP in acetonitrile (Fig. 6).
 | ||
| Fig. 6 The metal ion sensitivity profile for HQAP: the change in the absorbance of HQAP + 6.0 equivalents of the investigated interfering Mn+ + 2.0 equivalents of Ni2+. | ||
In the complex of HQAP and Ni2+ ion, the metal ion coordinates with the nitrogen atoms of 2-aminophenol and a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N Schiff group as well as the hydroxyl oxygen atom of the phenol moiety. On the basis of this binding mode, the ratiometric shift in the absorption spectra upon addition of the metal ion can be rationalized by ICT.
N Schiff group as well as the hydroxyl oxygen atom of the phenol moiety. On the basis of this binding mode, the ratiometric shift in the absorption spectra upon addition of the metal ion can be rationalized by ICT.
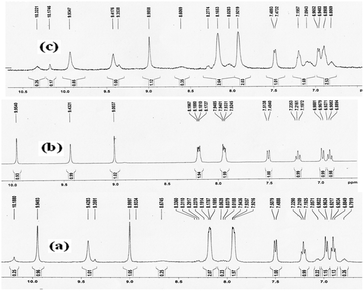
The coordination of a metal ion to the nitrogen and oxygen atom of phenol and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N Schiff moiety increases its electron-withdrawing character, which leads to a stronger ICT from the electron-donating hydroxyl group to the metal-complexed moiety. At the same time, due to the complexation process, the –OH proton of 2-aminophenol undergoes a downfield shift from δ 9.9540 ppm to δ 10.1746 ppm because the cationic species induces a downfield chemical shift through diamagnetic deshielding. Again noticeable downfield chemical shifts are also shown in the case of protons of quinoxaline-3CH and the imine proton of receptor HQAP from δ 9.4321 ppm to δ 9.9347 ppm and δ 9.0037 ppm to δ 9.4176 ppm respectively which are induced due to complexation after addition of 1.6 equivalents of nickel which is shown in the NMR titration curves (Fig. 7 and ESI†).
N Schiff moiety increases its electron-withdrawing character, which leads to a stronger ICT from the electron-donating hydroxyl group to the metal-complexed moiety. At the same time, due to the complexation process, the –OH proton of 2-aminophenol undergoes a downfield shift from δ 9.9540 ppm to δ 10.1746 ppm because the cationic species induces a downfield chemical shift through diamagnetic deshielding. Again noticeable downfield chemical shifts are also shown in the case of protons of quinoxaline-3CH and the imine proton of receptor HQAP from δ 9.4321 ppm to δ 9.9347 ppm and δ 9.0037 ppm to δ 9.4176 ppm respectively which are induced due to complexation after addition of 1.6 equivalents of nickel which is shown in the NMR titration curves (Fig. 7 and ESI†).
From an NMR study, we have investigated the molecular interactions between the receptor HQAP and nickel ions. The slightly downfield peak (δ 10.3221 ppm) probably belongs to the –OH of 2-aminophenol which decreased and the intensity of all the protons of the HQAP gradually increased after addition of 0.5 equivalents of nickel ions indicating that there is complex formation (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) between the –OH group of the 2-aminophenol moiety and nickel ions (Fig. 7). The selectivity of the receptor may be due to the enhanced acidity of the –OH of 2-aminophenol. The selectivity here is greatly influenced based on charge–transfer interactions between the metal and ligand and the involvement of both O–H⋯Ni bonds. The unique binding motif can find a greater utility in the development of new cation receptors/sensors with enhanced binding affinity and substrate specificity, which is actively being investigated.
1) between the –OH group of the 2-aminophenol moiety and nickel ions (Fig. 7). The selectivity of the receptor may be due to the enhanced acidity of the –OH of 2-aminophenol. The selectivity here is greatly influenced based on charge–transfer interactions between the metal and ligand and the involvement of both O–H⋯Ni bonds. The unique binding motif can find a greater utility in the development of new cation receptors/sensors with enhanced binding affinity and substrate specificity, which is actively being investigated.
In summary, we have developed a simple practically useful colorimetric chemosensor HQAP based on a quinoxaline moiety which exhibits highly selective and sensitive recognition towards Ni2+ ions in solution. The recognition of Ni2+ ions gave rise to a dramatic color change from colorless to deep violet in acetonitrile–methanol which was clearly visible to the naked eye. The detection limit of Ni2+ ions is as low as 4.16 μM in solution by the “naked eye” without resorting to any spectroscopic instrumentation. We believe that this economic chemosensor, with sensitive and selective naked eye responses can be used for many practical applications in chemical (laboratory practicals), environmental and biological systems. We also first proved the complex structure by single crystal X-ray diffraction.
Acknowledgements
We acknowledge the Department of Science and Technology (DST/SR/S1/OC-58/2010) and the Council of Scientific and Industrial Research (CSIR), Government of India, for financial support. S. C. thanks TCG Life sciences Ltd for their overall support. We also thank Dr. Kumaresh Ghosh of Kalyani University (formerly a Ph.D. student of our laboratory) for his assistance in checking the binding constants. The authors extend their appreciation to The Deanship of Scientific Research at King Saud University for funding the work through research group project no. RGP-VPP-207.Notes and references
- E. Denkhaus and K. Salnikow, Crit. Rev. Oncol. Hematol., 2002, 42, 35 CrossRef CAS.
- W. Lee, K. A. Davis, R. L. Rettmer and R. F. Lable, Am. J. Clin. Nutr., 1988, 48, 289 Search PubMed.
- X. Q. Liu, X. Zhou, X. Shu and J. Zhu, Macromolecules, 2009, 42, 7634 CrossRef CAS.
- J. R. Sheng, F. Feng, Y. Qiang, F. G. Liang, L. Sen and F. H. Wei, Anal. Lett., 2008, 41, 2203 CrossRef CAS.
- H. X. Wang, D. L. Wang, Q. Wang, X. Y. Li and C. A. Schalley, Org. Biomol. Chem., 2010, 8, 1017 CAS.
- L. Feng, Y. Zhang, L. Y. Wen, L. Chen, Z. Shen and Y. F. Guan, Analyst, 2011, 136, 4197 RSC.
- O. Haasw, M. Klarre, J. A. C. Broaekaert and K. K. Rothensee, Analyst, 1998, 123, 1219 RSC.
- C. E. C. Malgalhaes, F. J. Krug, A. H. Fostier and H. Berndt, J. Anal. At. Spectrom., 1997, 12, 1231 RSC.
- P. C. Rudner, A. G. Torres, J. M. C. Pavon and E. R. Castellon, J. Anal. At. Spectrom., 1998, 13, 243 RSC.
- F. Zapata, A. Caballero, P. Molina and A. Tarraga, Sensors, 2010, 10, 11311 CrossRef CAS PubMed.
- (a) D. Aldakov, M. A. Palacios and P. L. Anzenbacher Jr, Chem. Mater., 2005, 17, 5238 CrossRef CAS; (b) L. S. Hegedus, M. M. Greenberg, J. J. Wendling and J. P. Bullock, J. Org. Chem., 2003, 68, 4179 CrossRef CAS PubMed; (c) M. Sebastian, V. Arun, P. P. Robinson, P. Leeju, D. Varghese, G. Vaesha and K. K. M. Yusuff, J. Coord. Chem, 2010, 63, 307 CrossRef CAS; (d) S. Goswami, S. Chakroborty, S. Paul, S. Halder and A. C. Maity, Tetrahedron Lett., 2013, 54, 5075 CrossRef CAS PubMed.
- (a) X. He, Q. Zhang, X. Liu, L. Lin and X. Feng, Chem. Commun., 2011, 47, 11641 RSC; (b) L. Wang, D. Ye and D. Cao, Spectrochim. Acta, Part A, 2012, 90, 40 CrossRef PubMed; (c) U. El-Ayaan, F. Murata and Y. Fukuda, Monatsh. Chem., 2001, 132, 1279 CrossRef CAS; (d) N. Baho and D. Zargarian, Inorg. Chem., 2007, 46, 299 CrossRef CAS PubMed; (e) F. Averseng, P. G. Lacroix, I. Malfant, G. Lenoble, P. Cassoux and K. Nakatani, Chem. Mater., 1999, 11, 995 CrossRef CAS; (f) M. Ishida, Y. Naruta and F. Tani, Dalton Trans., 2010, 39, 2651 RSC; (g) H. Ohtsu and K. Tanaka, Inorg. Chem., 2004, 43, 3024 CrossRef CAS PubMed; (h) C. T. Chen, S. Y. Liao, K. J. Lin, C. H. Chen and T. Y. J. Lin, Inorg. Chem., 1999, 38, 2734 CrossRef CAS.
- B. Samanta, J. Chakraborty, S. Shit, S. R. Batten, P. Jensen, J. D. Masuda and S. Mitra, Inorg. Chim. Acta, 2007, 360, 2471 CrossRef CAS PubMed.
- A. A. Aziz and A. H. Kamel, Talanta, 2010, 80, 1356 CrossRef PubMed.
- F. A. Abebe, C. S. Eribal, G. Ramakrishna and E. Sinn, Tetrahedron Lett., 2011, 52, 5554 CrossRef CAS PubMed.
- Y. Wang and Z. Y. Yang, J. Lumin., 2008, 128, 373 CrossRef CAS PubMed.
- V. K. Gupta, A. K. Singh and M. K. Pal, Anal. Chim. Acta, 2008, 624, 223 CrossRef CAS PubMed.
- (a) A. A. Khandar, S. A. Hosseini-Yazdi, M. Khatamian and S. A. Zarei, Polyhedron, 2010, 29, 995 CrossRef CAS PubMed; (b) J. Fabian, H. Nakazumi and M. Matsuoka, Chem. Rev., 1992, 92, 1197 CrossRef CAS; (c) Y. Terazono, H. Hoshino and T. Yotsuyanagi, Chem. Lett., 1999, 28, 1251 CrossRef; (d) J. Chen, X. Wang, Y. Li and Z. Guo, J. Inorg. Biochem., 1996, 63, 265 CrossRef; (e) S. S. Mandal, U. Varshney and S. Bhattacharya, Bioconjugate Chem., 1997, 8, 798 CrossRef CAS PubMed.
- (a) S. Mayadevi and K. K. Mohammed Yusuff, Synth. React. Inorg. Met.-Org. Chem., 1997, 27(2), 319 CrossRef CAS; (b) S. Muvaffak and K. Seniz, Monatsh. Chem., 1976, 107, 1189 CrossRef.
- (a) S. P. Goswami and A. C. Maity, Chem. Lett., 2007, 36, 1118 CrossRef CAS; (b) S. P. Goswami and A. K. Adak, Synth. Commun., 2003, 33, 475 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 964936. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra00594e |
| This journal is © The Royal Society of Chemistry 2014 |