Magnetically recoverable and reusable CuFe2O4 nanoparticle-catalyzed synthesis of benzoxazoles, benzothiazoles and benzimidazoles using dioxygen as oxidant†
Abstract
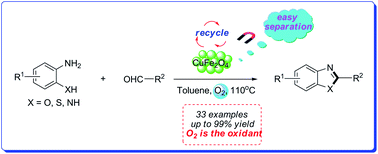
A green and efficient strategy for the synthesis of benzoxazoles, benzothiazoles and benzimidazoles has been developed by using inexpensive, readily available, dioxygen-stable and recyclable CuFe2O4 as the nanocatalyst, and o-substituted aminobenzene and various aldehydes as the starting materials. The CuFe2O4 nanoparticles are dioxygen insensitive and easily recoverable with an external magnet from the reaction medium. The catalyst can be reused ten times without significant loss of catalytic activity.

 Please wait while we load your content...
Please wait while we load your content...