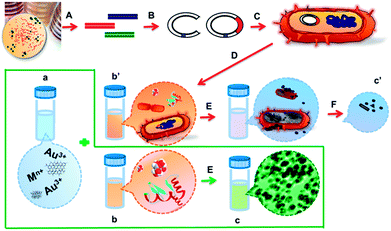
Are microorganisms indispensable in green microbial nanomaterial synthesis?†
Lihong Liu,
Zongping Shao,
Ha Ming Ang,
Moses O. Tadé and
Shaomin Liu*
Department of Chemical Engineering, Curtin University, Bentley, WA 6845, Australia. E-mail: Shaomin.Liu@curtin.edu.au; Fax: +61-8-92662681; Tel: +61-8-92669056
First published on 7th February 2014
Abstract
Typical microbial synthesis relies heavily on microbial alchemy. In this work, we demonstrate a conceptually new, greener strategy using aqueous microbial culture broths alone for nanosynthesis by eliminating the complication of manipulating genetically engineered microbes and maintaining the cell cultures. This versatile method is exemplified by the preparation of gold nanoparticles and reduced graphene oxide.
Most conventional methods of nanosynthesis can cause contamination, from precursor chemicals, organic solvents or hazardous by-products, which often raise environmental concerns.1 Hence, there is a significant benefit in developing nontoxic and environmentally benign processes for nanoparticle synthesis.2 It is well known that nanoparticles can be produced by either uni- or multi-cellular organisms or from agricultural biomass. These biological entities have successfully been used to produce biogenic nanomaterials such as magnetite,3 silica,4 gold,5 silver,6 and quantum dots.7 Although microbiological methods are considered to be environmentally-friendly, they require time-consuming microbe screening, plasmid construction and have a variable cell culture process.8 In addition, when scaling up, microbial nanoparticle synthesis cannot provide sufficient control over particle size, morphology and monodispersity. As the microorganisms can only tolerate a limited range of pH, temperature or toxic ion concentration, the synthesis conditions need to be very carefully controlled when living organisms are present in the broth. Moreover, in order to release the resultant intracellular nanoparticles, downstream operations like ultrasonic breakdown, detergent disintegration, high temperature calcination and freeze-thawing processes are normally required.9 These costly downstream processes are highly energy intensive and time consuming, which present a significant barrier when scaling up. To circumvent these limitations, extracellular nanoparticle synthesis has been attempted in cell-free extracts of certain special miroorganisms.10,11 For example, Das and co-workers have prepared multi-shaped gold nanoparticles using a protocol starting from a cultured fungal strain, Rhizopus oryzae, in a potato dextrose (PD) broth, and then separating the fungal biomass before employing a supernatant to reduce chloroauric acid. The composition of PD broth and other chemically defined media that have been frequently used for microbial nanosynthesis are listed in Table 1. Their common ingredients are found to be yeast extract, dextrose, peptone and other digested proteins, etc.
| Broth name | Dextrose | Yeast extract | Peptone | Other ingredients |
|---|---|---|---|---|
| a Malt extract.b Casein, soybean meal, NaCl and K2HPO4.c Tryptone and NaCl.d Potato extract.e Malt extract and CaCO3. | ||||
| Yeast mold | 10.0 | 3.0 | 5.0 | 3.0a |
| Tryptic soy broth | 2.5 | 27.5b | ||
| Lysogeny broth | 5.0 | 15.0c | ||
| Potato dextrose broth (ref. 10) | 20.0 | 200.0d | ||
| Streptomyces broth (ref. 27) | 4.0 | 4.0 | 12.0e | |
Yeast extract is concentrated from autolyzed Saccharomyces cerevisiae. S. cerevisiae cells have been employed to synthesize TiO2 nanoparticles12 and CdTe quantum dots7 in high yields. With its high content of B vitamins, tyrosine (1.2%, total amino acids13) and other unknown peptides, the yeast extract itself has the potential to reduce gold ions.14,15 Dextrose is a well-known agent for the green synthesis of AuNPs within a size range of ∼8–120 nm.16,17 Despite the progress achieved by employing these green reactants, conventional microbial studies rely heavily on manipulating the specific microbes rather than exploiting the microbe culture broths, and therefore, the potential of microbial nanoparticle synthesis is yet to be fully realized.
Here, we report a greener biosynthesis of gold nanoparticles and reduced graphene oxide in the absence of live microbes (as shown in Fig. 1). The effects of broth composition, pH value, precursor concentration and temperature on the nanoparticle formation have been carefully evaluated. More importantly, another objective of this work is to alert researchers to the fact that when the spent medium is employed in extra-cellular nanobiosynthesis, the synchronous reduction by dextrose, yeast extracts, vitamins, peptones and other digested proteins should not be ignored. A properly designed control experiment is vital to judge the necessity of microbial entities. Hopefully, this study will promote a deeper understanding of the biogenic nanomaterial formation mechanism.
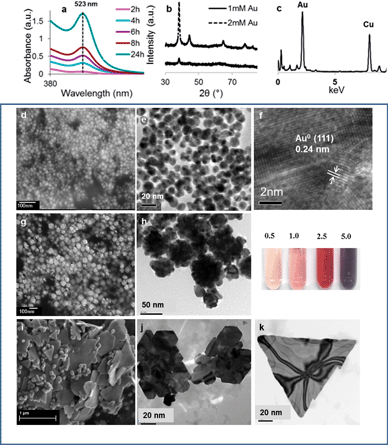
Among the precious metals, Au3+ has the largest positive standard reduction potential (E0 = 1.5 V) therefore has the greatest affinity to be reduced. We first evaluated the capability of the yeast mold (YM) broth in producing AuNPs when unaided by any recombinant microbes. The individual ingredients of various broths and the experimental procedures are described in detail in the ESI†. All broths were autoclaved at 121 °C for 15 min prior to further reaction. To study the pH-dependent reduction of the AuNPs, HCl or NaOH was added to get the desired pH values. In a typical experiment, 5 mL aqueous solution of HAuCl4 (2 mM) was combined with 5 mL YM broth at different pH levels. To mimic the in vivo microbial conditions, the reactions were performed at 37 °C in an orbital shaker (100 rpm). As expected, the colour of the solution changed gradually from light yellow to pink within 2 h due to the formation of AuNPs. The evolution of the AuNPs was recorded using time-course UV-vis spectroscopy (ESI Fig. S1†). In mildly basic broths (pH 8–10) the reaction was extremely slow. The broth at pH 6 offered faster particle growth; however, the AuNPs were not stable and aggregation occurred, indicated by the broadening and 15 nm red-shift of the surface plasmon resonance (SPR) bands over 18 h. At pH 12, the reaction was also accelerated and highly stable AuNPs were formed, as shown by the absorption peak centered at 523 nm (Fig. 2a). The particle size and size distribution of the resultant particles were investigated using dynamic light scattering (DLS, ESI Fig. S2†). The particle diameters at the reaction times of 4, 8 and 24 h were 18, 20 and 21 nm respectively and the corresponding polydispersity index (PDI) was 0.183, 0.150 and 0.145. The prepared nanoparticles (in pH 12 YM broth) exhibited excellent stability for at least 6 months under ambient conditions, including daily exposure to room light.
The precursor concentration has a profound influence on the size and morphology of AuNPs. By fixing the pH value at 12 but altering the [Au3+] from 0.5 to 5.0 mM, we found that the colour of the Au hydrosols, containing various concentrations of Au ions, was noticeably different (Fig. 2 inset). Increasing the concentration of the precursor led to a larger particle size. The AuNPs could be extracted by centrifugation at 8000 rpm and readily re-dispersed in deionised (DI) water by shaking the centrifuge tube by hand without obvious irreversible aggregation. The recovered AuNPs were analysed by XRD and TEM. Fig. 2b gives five peaks at 2θ of 38.2, 44.4, 64.6, 77.5 and 85, respectively, corresponding to (111), (200), (220), (311) and (222) Bragg reflections of face-centered cubic metallic gold.18
Facile particle synthesis at ambient temperature is more favourable for large scale application due to easy operation and reduced energy consumption compared to high temperature preparation. Therefore, we explored the feasibility of AuNP formation in a YM broth at room temperature (22 ± 3 °C). The growth of the AuNPs was confirmed using a series of UV-vis extinction spectra although the growth of the nanocrystals was relatively slow in comparison with the facilitated kinetics of the particle formation at 37 °C. AuNPs with the narrowest polydispersity (PDI = 0.048) were obtained as revealed in the TEM image (ESI Fig. S3†). Fig. 2c shows the energy dispersive spectroscopy (EDS) spectrum of the particles. A strong gold nanoparticle characteristic peak at 2.195 keV was clearly spotted. All of these data provide evidence to show that nanoparticles can be obtained under milder conditions. Noticeably, flower-shaped AuNPs (AuNFs) were observed by adjusting the initial [Au3+] to 2.5 mM. Several strategies focusing on multistep19 or template-mediation20–22 have been reported to synthesize gold nanoparticles with spike morphology. A facile, one-pot, template-free synthesis of flower-like AuNPs was reported by Xie's group.23 Interestingly, in this work, the anisotropic growth of the Au nanocrystals was also observed at high Au3+ concentration. Fig. 2h depicts the TEM image of AuNFs after 48 h reaction at room temperature. The particulates have a solid core with short spikes. The dimensions of the AuNF branches are 10 nm in diameter and 15 nm in length. The z-average diameter by DLS was 54.4, 70.6 and 86.3 nm at 4, 8, and 24 hours, respectively, with the SPR peaks at 556 nm. It is remarkable that Au nanoplates were formed after re-dispersing the harvested gold nanoparticles (synthesized using initial [Au3+] = 5 mM, room temperature and a 48 h incubation period) in ethanol. High-yield preparation of single-crystalline gold nanoplates has been achieved by heating a concentrated aqueous solution of linear polyethylenimine and HAuCl4 at 100 °C.24 Similarly, the formation of octahedral gold has been linked to the interaction of Au3+ with various microorganisms including bacteria,25 the cell-free extract of a fungus,10 and algae extracts.26 Our method features simple implementation and provides high yields of single-crystalline nanoplates.
Considerable efforts have been directed towards the understanding of the microbial nanoparticle synthesis mechanism. In general, the driving force of the particle formation is related to the detoxification of various microorganisms. Although it is unequivocally acknowledged that ATPase, especially the species-specific NADH-dependent nitrate reductase, contributes to nanoparticle reduction, the fundamental role of native enzymes continues to be debated within the scientific community. For example, no AuNPs were observed in the heat-denatured cell-free extract of the fungal strain Rhizopus oryzae;10 however, Au0 formation at appreciable rates was identified in the heat-treated cell-free filtrate of Trichoderma koningii.27 Based on the fact that the broths that we used were still active in producing NPs despite being autoclaved at 121 °C for 15 min, we support the latter suggestion that native enzyme structures do not play an indispensable role in the biogenic process.
Based on previous reports, we speculate that abundant reducing and capping agents in the broth, like dextrose and S. cerevisiae yeast extract, are capable of synthesizing metal nanoparticles without the involvement of living organisms. To verify this hypothesis, we tested the possibility of using pure dextrose, yeast extract (YE) and peptone to reduce Au ions, since they are the common ingredients in several of the defined nutrient broths. As shown in Fig. S4 and S5,† Au ions were successfully reduced by these respective biological molecules. It is of note that dextrose with an alkaline pH range demonstrated a faster reducing rate, compared to previous reactions at pH 7. At neutral pH, it took 1–6 h to hours to complete the reduction under mild conditions.17 Surprisingly, Au NPs were formed in seconds without agitating the dextrose solution at pH 12 (ESI video†). The comparison of FTIR spectra (Fig. S6†) between pure dextrose and AuNPs reduced in pH 12 dextrose (0.25% in weight) reveals the close association between the hydroxyl group of dextrose and the Au NP surface (the strong absorption band of the –OH stretching mode shifted from 3251 to 3407 cm−1). This resultant fast production rate is highly beneficial when scaling up nanoparticle synthesis. Unlike other chemical methods that can realize rapid nanoparticle formation in continuous flow reactors,28 the large-scale biological synthesis of nanoparticles has been restricted by a slow reaction rate. In this context, the discovery that dextrose can be used as an extremely strong reducing agent for immediate nanoparticle synthesis provides an ideal candidate for continuous operation mode.
The AuNP formation in yeast extract (YE) was slower compared to the dextrose (pH 12) reduction. After stirring the mixture at room temperature for 3 days, the average AuNP size was 44 and 200 nm when the YE concentration increased from 0.5% to 1% in weight. Interestingly, a black gold colloidal solution was formed in 1% YE solution after a 24 h reaction. A similar phenomenon was observed when a secondary metabolite secreted by Delftia acidovorans was incubated with AuCl3.4 The authors demonstrated that a nonribosomal peptide generated the AuNPs. In this study, we assume peptide bonds from the YE might lead to the growth of the AuNPs.
FTIR spectroscopic measurements were carried out to detect the functional groups contributing to the AuNP synthesis in the YM broth. The major functional groups of pure yeast extract at 1579 cm−1 (amide II, N–H and C–H vibrations of the peptide bonds in different protein conformations) and 1399 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of COO– symmetric stretching in proteins)29 were observed in the centrifuged and washed AuNPs reflecting the bonds of amino acids (Fig. S7†). These results confirmed the critical roles played by the YE and dextrose as efficient reducing and stabilizing agents.
O of COO– symmetric stretching in proteins)29 were observed in the centrifuged and washed AuNPs reflecting the bonds of amino acids (Fig. S7†). These results confirmed the critical roles played by the YE and dextrose as efficient reducing and stabilizing agents.
The synthesis of AuNPs in other typical microbial culture broths was performed to further confirm the proposed formation mechanism. Lysogeny broth (LB) is one of the most common media for cultivating recombinant strains of E. coli.9 Tryptic soy broth (TSB) is another general purpose medium for the cultivation of a wide variety of microorganisms. Again, stable Au nanoparticles were formed in LB and TSB at pH 12. Although the same experimental conditions were used for these broths, the gold sol formation was drastically slower in the LB and TSB broths, compared to the YM broth. The broth dependence of AuNP formation rate may be explained by the decreasing dextrose concentration in YM, TSB and LB (1%, 0.25% and 0%, respectively). Similarly, in a biosynthesis study of Au and Ag NPs by a novel marine strain of Stenotrophomonas, the authors found the particles were formed in the streptomyces broth (SB) but not in the luria broth (LB).30 Although the SDS-PAGE analysis of the extracellular media supernatant revealed three low molecular weight proteins (∼36.9, ∼17.6 and ∼14.9 kDa) in SB, suggesting a potential involvement of these secretory proteins in the particle synthesis, the important contribution from dextrose and reduced sugars (maltoses) (0.4% and ∼1.0%, respectively in SB only) should also be considered to understand the nanobiosynthesis mechanism better.
With all these concerns, we propose a new formation mechanism of gold nanoparticles in pH 12 broth containing dextrose as follows. Similar to the reduction of Au3+ by NaBH4 and the stabilization by sodium citrate, the growth process starts with a rapid reduction by dextrose followed by a nucleation process of gold clusters which undergo coalescence processes until the final size is reached, the process of protein stabilization.
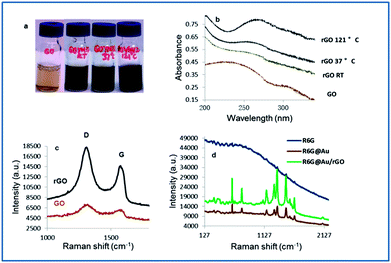
In order to extend the application of graphene from electronic devices to biomedical fields, a few green strategies have been attempted recently to reduce graphene oxide (GO) with fewer contaminants.31 The Shewanella species represent an important family of GO-reducing bacteria.32 An extracellular electron transfer (EET) network was suggested to have involved the GO reduction.33 We demonstrate here that the YM broth (pH 12) alone could reduce GO even at room temperature, although a higher reduction rate was achieved in 15 min at 121 °C in an autoclave, as observed by the colour change of the graphene oxide dispersion. The as prepared sample was characterized systematically using the UV-vis spectra to confirm the formation of an rGO sheet. As shown in Fig. 3b, the absorption maximum at about 230 nm, due to the π → π* transition of aromatic C–C bonds in GO, was found to continuously shift to about 260 nm with the increase in reduction temperature, suggesting the restoration of the π-conjugation network within the graphene sheets.34 Raman spectroscopy reveals that rGO has much narrower Raman peaks than the sample. Moreover, an increase in the intensity ratio of the D band (located at 1298 cm−1) and G band (located at 1574 cm−1) was observed, indicating that the new domains of the conjugated carbon atoms (bonded with sp2 hybridization) were formed accompanying the removal of the oxygen containing group, which is consistent with previous reports on GO reduction.35,36
The AuNP decorated rGO has become a new class of surface enhanced Raman scattering (SERS) substrate for the trace detection of heavy metal ions.37,38 A one-pot facile synthesis of Au/rGO nanocomposites is realized by employing our strategy. A comparison of the Raman spectra of Rhodamine 6G chloride (R6G) only (in powder form), R6G on AuNPs and R6G on Au/rGO, revealed that the enhanced Raman vibrations of the R6G molecules corresponded to the Raman fingerprints in the 1200–1700 cm−1 region (Fig. 3d). The Au/rGO substrate exhibited about a 3-fold increase in SERS intensity compared to the AuNPs.
Based on microbial green chemistry perspectives, material scientists are focusing more on nanosynthesis, using specific organisms as nanofactories. According to our knowledge, we are the first to take the microbial approach a step further, to employ a commercially available broth as a starting material to prepare multi-shaped gold nanomaterials as well as a semiconductor material like reduced graphene oxide. Although the reduction mechanism seems straightforward, the results of this study highlight the important synchronous reduction by various broths that have been ignored for a long time.
Conclusions
In this study, we have presented a new concept for the greener synthesis of gold nanoparticles and reduced graphene oxide by using chemically defined microbe culture media alone, without the involvement of microbes. Thus, the requirement of highly laborious microorganism screening, cultivating and the complex down-stream separation processing can be eliminated. The capability to flexibly tune the particle size and morphology adds an extra advantage to this methodology. We are hereby advocating a more straightforward and economically viable approach by minimizing the use and generation of substances and minimizing the energy/time of the process, which are in line with the twelve principles of green chemistry formulated by Paul Anastas and John Warner. We are also using this article to alert researchers in the microbial nanosynthesis field to carefully design control experiments to avoid the misunderstanding of the microbial synthesis mechanisms and realize the implementation of complete green methods to fabricate technologically important nanomaterials.Acknowledgements
This work was financially supported by the Australian Research Council (DP110104599). Some of this research was carried out using facilities at the University of Western Australia's Centre for Microscopy, Characterisation and Analysis, and at Curtin University, which are supported by university, state, and federal government funding. We thank Dr Robert Hart and Dr Xiaodong Wang for invaluable support in TEM characterization. The authors acknowledge the helpful comments on the manuscript by Dr Scott Battersby.Notes and references
- J. A. Dahl, B. L. S. Maddux and J. E. Hutchison, Chem. Rev., 2007, 107, 2228–2269 CrossRef CAS PubMed.
- M. A. Faramarzi and A. Sadighi, Adv. Colloid Interface Sci., 2013, 189–190, 1–20 CrossRef CAS PubMed.
- D. R. Lovley, J. F. Stolz, G. L. Nord Jr and E. J. P. Phillips, Nature, 1987, 330, 252–254 CrossRef CAS.
- N. Kröger, S. Lorenz, E. Brunner and M. Sumper, Science, 2002, 298, 584–586 CrossRef PubMed.
- C. W. Johnston, et al., Nat. Chem. Biol., 2013, 9, 241–243 CrossRef CAS PubMed.
- P. Mukherjee, et al., Nano Lett., 2001, 1, 515–519 CrossRef CAS.
- H. Bao, N. Hao, Y. Yang and D. Zhao, Nano Res., 2010, 3, 481–489 CrossRef CAS PubMed.
- J. H. Jung, T. J. Park, S. Y. Lee and T. S. Seo, Angew. Chem., Int. Ed., 2012, 51, 5634–5637 CrossRef CAS PubMed.
- T. J. Park, S. Y. Lee, N. S. Heo and T. S. Seo, Angew. Chem., Int. Ed., 2010, 49, 7019–7024 CrossRef CAS PubMed.
- S. K. Das, A. R. Das and A. K. Guha, Small, 2010, 6, 1012–1021 CrossRef CAS PubMed.
- A. Ahmad, S. Senapati, M. I. Khan, R. Kumar and M. Sastry, Langmuir, 2003, 19, 3550–3553 CrossRef CAS.
- K. Jha, K. Prasad and A. R. Kulkarni, Colloids Surf., B, 2009, 71, 226–229 CrossRef PubMed.
- BD Bionutrients™ Technical Manual, Third Edition Revised.
- M. N. Nadagouda and R. S. Varma, Green Chem., 2006, 8, 516–518 RSC.
- A. Swami, A. Kumar, M. D'Costa, R. Pasricha and M. Sastry, J. Mater. Chem., 2004, 14, 2696–2702 RSC.
- J. Liu, G. Qin, P. Raveendran and Y. Ikushima, Chem.–Eur. J., 2006, 12, 2131–2138 CrossRef CAS PubMed.
- V. D. Badwaik, et al., Langmuir, 2011, 27, 5549–5554 CrossRef CAS PubMed.
- P. Mukherjee, et al., Angew. Chem., Int. Ed., 2001, 40, 3585–3588 CrossRef CAS.
- L. Lu, K. Ai and Y. Ozaki, Langmuir, 2008, 24, 1058–1063 CrossRef CAS PubMed.
- Z. Wang, J. Zhang, J. M. Ekman, P. J. A. Kenis and Y. Lu, Nano Lett., 2010, 10, 1886–1891 CrossRef CAS PubMed.
- T. K. Sau, A. L. Rogach, M. Döblinger and J. Feldmann, Small, 2011, 7, 2188–2194 CrossRef CAS PubMed.
- A. Mohanty, N. Garg and R. Jin, Angew. Chem., Int. Ed., 2010, 49, 4962–4966 CrossRef CAS PubMed.
- J. Xie, Q. Zhang, J. Y. Lee and D. I. C. Wang, ACS Nano, 2008, 2, 2473–2480 CrossRef CAS PubMed.
- X. Sun, S. Dong and E. Wang, Langmuir, 2005, 21, 4710–4712 CrossRef CAS.
- M. F. Lengke, M. E. Fleet and G. Southam, Langmuir, 2006, 22, 2780–2787 CrossRef CAS PubMed.
- J. Xie, J. Y. Lee, D. I. C. Wang and Y. P. Ting, Small, 2007, 3, 672–682 CrossRef CAS PubMed.
- I. Maliszewska, Ł. Aniszkiewicz and Z. Sadowski, Proceedings of the III National Conference on Nanotechnology NANO, 2009 Search PubMed.
- X. Z. Lin, A. D. Terepka and H. Yang, Nano Lett., 2004, 4, 2227–2232 CrossRef CAS.
- M. Cavagna, R. Dell'Anna, F. Monti, F. Rossi and S. Torriani, J. Agric. Food Chem., 2010, 13, 39–45 CrossRef PubMed.
- A. Malhotra, K. Dolma, N. Kaur, Y. S. Rathore, A. S. Mayilraj and A. R. Choudhury, Bioresour. Technol., 2013, 142, 727–731 CrossRef CAS PubMed.
- J. Gao, F. Liu, N. Ma, Z. Wang and X. Zhang, Chem. Mater., 2010, 22, 2213–2218 CrossRef CAS.
- E. C. Salas, Z. Sun, A. Lüttge and J. M. Tour, ACS Nano, 2010, 4, 4852–4856 CrossRef CAS PubMed.
- G. Wang, F. Qian, C. Saltikov, Y. Jiao and Y. Li, Nano Res., 2011, 4, 563–570 CrossRef CAS PubMed.
- Z. Lei, L. Lu and X. Zhao, Energy Environ. Sci., 2012, 5, 6391–6399 CAS.
- S. Park, J. An, I. Jung, R. Piner, S. An, X. Li, A. Velamakanni and R. Ruoff, Nano Lett., 2009, 9, 1593–1597 CrossRef CAS PubMed.
- H. Feng, R. Cheng, X. Zhao, X. Duan and J. Li, Nat. Commun., 2013, 4, 1539–1545 CrossRef PubMed.
- J. Huang, L. Zhang, B. Chen, N. Ji, F. Chen, Y. Zhang and Z. Zhang, Nanoscale, 2010, 2, 2733–2738 RSC.
- X. Ding, L. Kong, J. Wang, F. Fang, D. Li and J. Liu, ACS Appl. Mater. Interfaces, 2013, 5, 7072–7078 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra00555d |
| This journal is © The Royal Society of Chemistry 2014 |