The luminescence properties of novel α-Mg2Al4Si5O18:Eu2+ phosphor prepared in air
Abstract
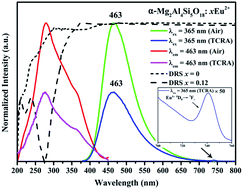
The α-Mg2Al4Si5O18:Eu2+ phosphor was firstly prepared via the conventional high temperature solid-state reaction method and the reduction of Eu3+ to Eu2+ in air was observed in α-Mg2Al4Si5O18:Eu. The phase structure, photoluminescence (PL) properties, the PL thermal stability and the fluorescence decay curves of the samples were investigated, respectively. Emission and excitation spectra were employed to detect the presence of Eu2+ ions in the compound. Under the excitation at 365 nm, the phosphor exhibited a broad-band blue emission with peak at 463 nm, which was ascribed to the 4f–5d transition of Eu2+. It was further proved that the dipole–dipole interactions resulted in the concentration quenching of Eu2+ in α-Mg2Al4Si5O18:xEu2+ phosphors. When the temperature was increased to 150 °C, the emission intensity of the α-Mg2Al4Si5O18:0.12Eu2+ phosphor was 59.07% of the initial value at room temperature. The activation energy ΔE was calculated to be 0.21 eV, which proved the good thermal stability of the sample. All the properties indicated that the blue-emitting α-Mg2Al4Si5O18:Eu2+ phosphor has potential application in white LEDs.

 Please wait while we load your content...
Please wait while we load your content...