One-pot synthesis of cuboid WO3 crystal and its gas sensing properties
Chong Wanga,
Xin Lia,
Biao Wangb,
Jian Maa,
Yang Caoa,
Yanfeng Sun*a and
Geyu Lu*a
aState Key Laboratory on Integrated Optoelectronics, College of Electronic Science and Engineering, Jilin University, Changchun 130012, People's Republic of China. E-mail: syf@jlu.edu.cn; lugy@jlu.edu.cn; Fax: +86 431 85167808; Tel: +86 431 85167808
bState Key Laboratory of Luminescence and Application, Changchun Institute of Optics, Fine Mechanics and Physics, Chinese Academy of Sciences, Changchun 130033, China
First published on 16th April 2014
Abstract
In this work, a simple solvothermal method was used for the synthesis of cuboid WO3 crystals without any surface active agent. The calcined WO3 was characterized using field emission scanning electron microscopy (FESEM), X-ray powder diffraction (XRD) and transmission electron microscopy (TEM). The results indicate that the sample is composed of cuboid nanobulks. The gas sensing property of a sensor based on the cuboid WO3 was also investigated. It is found that the sensor has a high response to low concentration NO2 at low operation temperature, so the cuboid nanobulks might have a potential application in the fabrication of highly sensitive and low power consumption NO2 gas sensors.
1. Introduction
With the increasing attention on atmospheric pollution, the detection of harmful gases at low concentrations becomes an important issue. So, a lot of effort has been devoted to the exploration of new material with enhanced gas sensing performance in recent years. The existing gas sensor materials include semiconducting metal oxides,1,2 silicon,3,4 organic5,6 and fiber optic chemical materials.7,8 Among them, semiconducting metal oxides have gained much more focus due to their superior properties. Up to now, a variety of semiconductor nanomaterials have been successfully synthesized, such as ZnO,9,10 SnO2,11,12 CuO,13,14 Fe2O3 (ref. 15 and 16) and In2O3.17,18 As is well known, NO2 is a highly harmful gas among the toxic gases, causing acid rain and photochemical smog, and further affecting people's health, even their lives. WO3 is found to show a high response to NO2 with high selectivity.19,20 However, achieving a high response to ppb levels of NO2 is still a challenge.In order to improve the performance of the sensors based on WO3, various structures with different morphologies have been investigated: nanoplates,21 nanorods,22 nanospheres,23 nanoflowers24 and other hierarchically complex architectures. At the same time, different physical and chemical synthetic methods have also been explored to obtain WO3 crystals.25–28 Among all these methods, the solvothermal method is considered a simple and practicable operation to synthesize high yield crystals with good monodispersity. Moreover, this low-cost synthesis is also a key point of researchers' pursuit all the time. Consequently, it is strongly desirable to develop a simple, effective, and economical approach for the synthesis of WO3 with excellent NO2 sensing performance.
Herein, we report a facile method for the preparation of WO3 crystals by a simple process. Further, sensing performance based on the calcined WO3 nanocrystal is also investigated here. It is found that the sensor showed a high response to ppb levels of NO2 at 100 °C.
2. Experimental
2.1. Synthesis and characterization of WO3 nanobulks
All the reagents (analytical-grade purity) were used without any further purification. In a typical synthesis, 10 mL glycerol and 25 mL distilled water were mixed together by stirring. One hour later, 0.66 g sodium tungstate was added to the solution to form a homogeneous solution. Then, 2.5 mL 12 M HCl was dropped to the sodium tungstate solution, and yellow precipitates formed. Five minutes later, the above solution was transferred into a Teflon-lined stainless steel autoclave, sealed tightly, and maintained at 180 °C for 12 h. After the autoclave was cooled to room temperature naturally, the precipitates were washed with deionized water and absolute ethanol several times using centrifuge, and then dried at 80 °C for 24 h. The precipitates were calcined at 500 °C for 2 h with a heating rate of 2 °C min−1. The calcined products were then collected for further analysis.X-ray power diffraction (XRD) analysis was conducted on a Rigaku D/max-2500 X-ray diffractometer with Cu Kα1 radiation (λ = 1.54056 Å) in the range of 20–60°. The morphology was examined by field-emission scanning electron microscopy (FESEM, JEOL JSM-7500F, operated at an acceleration voltage of 15 kV). Transmission electron microscopy (TEM) and selected-area electron diffraction (SAED) were obtained on a JEOL JEM-2100 microscope operated at 200 kV.
2.2. Fabrication and measurement of sensor
The calcined powders were mixed with deionized water to form a paste, which was then coated onto an alumina tube (4 mm in length, 1.2 mm in external diameter and 0.8 mm in internal diameter) using a small brush, slowly and lightly. The tube was installed with a pair of gold electrodes, and each electrode was connected with two Pt wires. After brushing, a thick film was formed. After drying at room temperature, the sensing device was then sintered at 300° for 2 h in air. Finally, a Ni–Cr alloy coil was inserted into the alumina tube as a heater in order to control the operating temperature of the sensor. A schematic structure of the as-fabricated sensor is shown in Fig. 1a.The gas sensing property of the sensor was measured by a static process, and the schematic diagram is given in Fig. 2b. The resistance in the chamber (the volume is 50 L) filled with air is Ra, and then a certain amount of target gas is injected into this closed chamber. When the resistance reached Rg, the gas is discharged with the help of an air blower and fan. The data are collected by digital precision multimeter every second. S = Rg/Ra is defined as the response of the sensor for oxidizing gas and Ra/Rg for reducing gas; here, Ra and Rg is the resistance of the sensor in the air and target gas.
3. Results and discussion
3.1. Structural and morphological characteristics of the calcined WO3
The X-ray diffraction (XRD) patterns of the calcined products are shown in Fig. 2. It can be seen from Fig. 2 that all of the diffraction peaks can be well indexed to the standard WO3 (JCPDS file no. 83-950). No other diffraction peaks from any impurities are observed, indicating the high purity of the product. The XRD patterns (not shown here) of the sample without calcination are almost the same as the calcined products.The morphology of the calcined product was investigated by field emission scanning electron microscopy (FESEM). The typical SEM images are shown in Fig. 3a and b. As can be seen from Fig. 3a and b, the products are mostly composed of cuboid three-dimensional nanobulks. The TEM image is shown in Fig. 3c; an individual typical cuboid three-dimensional nanobulk is also observed, which agrees with the SEM results. The selected area electron diffraction pattern (SAED) was also performed on the calcined products, as shown in Fig. 3d, which confirmed that the nanobulks are single crystalline monoclinic WO3.
 | ||
| Fig. 3 (a and b) Typical SEM images of the calcined product. (c) TEM image of the WO3 cubic nanobulks. (d) The corresponding SAED pattern. | ||
3.2. Gas sensing properties for NO2
The sensing properties of a sensor based on the as-prepared WO3 samples were investigated. In order to determine the optimum operating temperature, the response of the sensor to 80 ppb NO2 was measured at different temperatures. As shown in Fig. 4, the response of the sensor increases from 75 °C to 100 °C. When the temperature further increases above 100 °C, the response begins to decrease gradually. This phenomenon could be explained as follows: at low temperatures (between 75 °C and 100 °C), the increase of the response can be attributed to the increase of the surface reaction (NO2(g) + e− → NO2−(ads)). Such adsorption can capture the electrons from WO3, resulting in the increased electrical resistance. However, when the temperature is higher (between 100 °C and 200 °C), the chemi-adsorbed oxygen becomes larger and plays a significant role (O(ads)2− + NO2 → NO2−(ads) + e−), and this kind of reaction goes against the adsorption of NO2. On the other hand, the rate of adsorption is lower compared to desorption at the higher temperature, which might be another reason for the decrease of the sensor's response.35–37 Accordingly, at the temperature of the maximum response, the optimum balance between adsorption and desorption has been established for the NO2 molecules. Therefore, the optimal working temperature of 100 °C is chosen to further examine the characteristics of the gas sensor.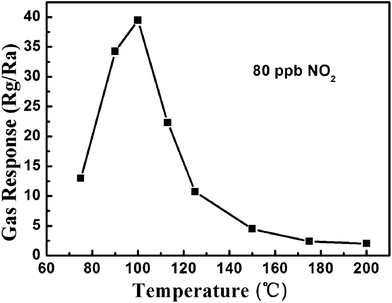 | ||
| Fig. 4 Correlations between the gas response and response time to 80 ppb NO2 and the operating temperature for the sensor using the as-obtained WO3. | ||
The response transient curve of the sensor to stepwise increase of the NO2 concentration from 0 to 160 ppb at optimum operating temperature is shown in Fig. 5a. The inset is the resistance transient in the presence of only 80 ppb of NO2. It is obvious that the resistance increases when exposed to gradually increased concentrations of NO2. In other words, the response increases with increasing concentration. The relationship between the response of the sensor and the concentration of NO2 is shown in Fig. 5b; the error bars are also displayed. The error bars represent the standard deviation at a fixed concentration, and they are small in all cases, which means a low experimental variability. It is worth noting that this sensor responds to low concentrations of NO2, and even the 10 ppb NO2 can also be detected. The response transients of the sensor to 160 ppb NO2 was measured at 100 °C (Fig. 6). The four reversible cycles of the response curve indicate a stable and repeatable characteristic, as shown in the inset of Fig. 6. A comparison between the sensing performances of the sensor fabricated in our work and in literature reports is summarized in Table 1. From the comparison, it can be seen that the sensor based on cuboid WO3 crystals has a correspondingly high response and low operating temperature. The high response of this cuboid nanobulk material may be attributed to the following reasons. On one hand, the product has a good crystalline character. The high crystallization will result in low bulk defects, thus lowering the conductivity of the sensing body. Due to this reason, only a small amount of NO2 adsorption will cause the resistance to change greatly. The conductivity of the sensor with the variation of operating temperature is also shown in Fig. 7; it can be clearly observed that the conductivities increase rapidly between 50 °C and 75 °C, and then increase slowly between 75 °C and 200 °C. On the other hand, the cuboid structure has trim edges, which will form grain boundaries in the sensing body. According to Li et al.33 and Hyodo et al.,34 grain boundaries or grain junctions are considered as the active sites, and they act positively on the sensor response. Therefore, the sensor is expected to have a high response to NO2.
 | ||
| Fig. 5 (a) Response transients of the sensor to different NO2 concentration at 100 °C. (b) Gas response of the sensor as a function of NO2 concentrations. Error bars are the standard deviations. | ||
 | ||
| Fig. 6 Response transients of the sensor to 160 ppb NO2 at 100 °C, the inset displaying four periods of response curve. | ||
It is well known that selectivity is another important parameter of a sensor in view of its practical application; therefore, at the optimum operating temperature, the response of the cuboid nanobulk-based sensor to various kinds of gases, such as Cl2, CO, H2S, NH3, acetone and ethanol, was investigated. The results are shown in Fig. 8 with error bars. It can be seen that the sensor has a high response to NO2 compared to the other gases. Such result demonstrates that the sensor using the WO3 nanostructure synthesized here exhibits an excellent selectivity to NO2 against the other tested gases at the working temperature of 100 °C.
 | ||
| Fig. 8 Comparison of responses of the sensor based on WO3 to various gases at 100 °C. Error bars represent the variability obtained in measurements. | ||
4. Conclusion
In summary, cuboid WO3 crystal has been successfully synthesized through a simple one-step solution route combined with a subsequent calcining process. Field emission scanning electron microscopy and transmission electron microscopy results demonstrate that the products are composed of nanobulks and that they are single crystal. In addition, the gas sensing properties of the calcined WO3-based sensor toward NO2 were investigated. The sensor based on the cuboid WO3 nanobulks exhibits excellent NO2 sensing properties at 100 °C. In particular, the response to 40 ppb reaches 23 at 100 °C. These results indicate that our sensor might have potential application in fabricating highly sensitive and low power consumption NO2 gas sensor devices.Acknowledgements
This work is supported by Application and Basic Research of Jilin Province (20130102010JC), the National Nature Science Foundation of China (no. 61304242, 61327804, 61134010 and 61374218), Program for Chang Jiang Scholars and Innovative Research Team in University (no. IRT13018) and “863” High Technology Project (2013AA030902).References
- N. Yamazoe, G. Sakai and K. Shimanoe, Catal. Surv. Asia, 2003, 7, 63–75 CrossRef CAS.
- N. Yamazoe, Sens. Actuators, B, 1991, 5, 7–19 CrossRef CAS.
- S. E. Lewis, J. R. DeBoer, J. L. Gole and P. J. Hesketh, Sens. Actuators, B, 2005, 110, 54–65 CrossRef CAS PubMed.
- L. Boarino, C. Baratto, F. Geobaldo, G. Amato, E. Comini, A. M. Rossi, G. Faglia, G. Lérondel and G. Sberveglieri, Mater. Sci. Eng., B, 2000, 69-70, 210–214 CrossRef.
- O. S. Wolfbeis, Anal. Chem., 2006, 78, 3859–3874 CrossRef CAS PubMed.
- R. C. Jorgenson and S. S. Yee, Sens. Actuators, B, 1993, 12, 213–220 CrossRef CAS.
- L. Torsi, A. Dodabalapur, L. Sabbatini and P. G. Zambonin, Sens. Actuators, B, 2000, 67, 312–316 CrossRef CAS.
- K. H. An, S. Y. Jeong, H. R. Hwang and Y. H. Lee, Adv. Mater., 2004, 16, 1005–1009 CrossRef CAS.
- B. B. Rao, Mater. Chem. Phys., 2000, 64, 62–65 CrossRef CAS.
- S. Roy and S. Basu, Bull. Mater. Sci., 2002, 25, 513–515 CrossRef CAS.
- J. Watson, Sens. Actuators, B, 1984, 5, 29–42 CrossRef CAS.
- A. Maiti, J. A. Rodriguez, M. Law, P. Kung, J. R. McKinney and P. D. Yang, Nano Lett., 2003, 3, 1025–1028 CrossRef CAS.
- J. T. Zhang, J. F. Liu, Q. Peng, X. Wang and Y. D. Li, Chem. Mater., 2006, 18, 867–871 CrossRef CAS.
- Y. S. Kim, I. S. Hwang, S. J. Kim, C. Y. Lee and J. H. Lee, Sens. Actuators, B, 2008, 135, 298–303 CrossRef CAS PubMed.
- J. Chen, L. Xu, W. Li, X. Gou, J. Chen, L. Xu, W. Li and X. Gou, Adv. Mater., 2005, 17, 582–586 CrossRef CAS.
- P. Sun, Y. W. Liu, X. W. Li, Y. F. Sun, X. S. Liang, F. M. Liu and G. Y. Lu, RSC Adv., 2012, 2, 9824–9829 RSC.
- T. Waitz, T. Wagner, T. Sauerwald, C. D. Kohl and M. Tiemann, Adv. Funct. Mater., 2009, 19, 653–661 CrossRef CAS.
- X. M. Xu, X. Li, W. B. Wang, B. Wang, P. Sun, Y. F. Sun and G. Y. Lu, RSC Adv., 2014, 4, 4831–4835 RSC.
- X. L. Li, T. J. Lou, X. M. Sun and Y. D. Li, Inorg. Chem., 2004, 43, 5442–5449 CrossRef CAS PubMed.
- B. B. Cao, J. J. Chen, X. J. Tang and W. L. Zhou, J. Mater. Chem., 2009, 19, 2323–2327 RSC.
- J. M. Ma, J. Zhang, S. R. Wang, T. H. Wang, J. B. Lian, X. C. Duan and W. J. Zheng, J. Phys. Chem. C, 2011, 115, 18157–18163 CAS.
- J. M. Wang, E. Khoo, P. S. Lee and J. Ma, J. Phys. Chem. C, 2008, 112, 14306–14312 CAS.
- Q. J. Sun, J. M. Luo, Z. F. Xie, J. D. Wang and X. T. Su, Mater. Lett., 2008, 62, 2992–2994 CrossRef CAS PubMed.
- J. R. Huang, X. J. Xu, C. P. Gu, G. J. Fu, W. Z. Wang and J. H. Liu, Mater. Res. Bull., 2012, 47, 3224–3232 CrossRef CAS PubMed.
- J. Zhou, Y. Ding, S. Z. Deng, L. G. Ning, S. Xu and Z. L. Wang, Adv. Mater., 2005, 17, 2110–2114 CrossRef.
- Y. Djaoued and S. Balaji, J. Sol-Gel Sci. Technol., 2013, 65, 374–383 CrossRef CAS.
- J. Zhang, J. P. Tu, X. H. Xia, X. L. Wang and C. D. Gu, J. Mater. Chem., 2011, 21, 5492–5498 RSC.
- A. Phuruangrat, D. J. Ham, S. J. Hong, S. Thongtem and J. S. Lee, J. Mater. Chem., 2010, 20, 1683–1690 RSC.
- T. Kida, A. Nishiyama, M. Yuasa, K. Shimanoe and N. Yamazoe, Sens. Actuators, B, 2009, 135, 568–574 CrossRef CAS PubMed.
- A. Ponzoni, E. Comini, M. Ferroni and G. Sberveglieri, Thin Solid Films, 2005, 490, 81–85 CrossRef CAS PubMed.
- E. Rossinyol, A. Prim, E. Pellicer, J. Arbiol, F. H. Ramírez, F. Peiró, A. Cornet, J. R. Morante, L. A. Solovyov, B. Tian, T. Bo and D. Y. Zhao, Adv. Funct. Mater., 2007, 17, 1801–1806 CrossRef CAS.
- Y. X. Qin, M. Hu and J. Zhang, Sens. Actuators, B, 2010, 150, 339–345 CrossRef CAS PubMed.
- E. Li, Z. X. Cheng, J. Q. Xu, Q. Y. Pan, W. J. Yu and Y. L. Chu, Cryst. Growth Des., 2009, 9, 2146–2151 CAS.
- T. Hyodo, N. Nishida, Y. Shimizu and M. Egashira, Sens. Actuators, B, 2002, 83, 209–215 CrossRef CAS.
- G. Lu, N. Miura and N. Yamazoe, Ionics, 1998, 4, 16–24 CrossRef CAS.
- X. Q. An, J. C. Yu, Y. Wang, Y. M. Hu, X. L. Yu and G. J. Zhang, J. Mater. Chem., 2012, 22, 8525–8531 RSC.
- A. P. Lee and B. J. Reedy, Sens. Actuators, B, 1999, 60, 35–42 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |



