IBX works efficiently under solvent free conditions in ball milling†
Tapas Kumar Achar,
Saikat Maiti and
Prasenjit Mal*
School of Chemical Sciences, NISER Bhubaneswar, IOP Campus, PO Sainik School, Bhubaneswar, Odisha 751005, India. E-mail: pmal@niser.ac.in; Fax: +91-6742302436
First published on 24th February 2014
Abstract
IBX (2-iodoxybenzoic acid), discovered in 1893, is an oxidant in synthetic chemistry whose extensive use is impeded by its explosiveness at high temperature and poor solubility in common organic solvents except DMSO. Since the discovery of Dess–Martin Periodinane in 1983, several modified IBX systems have been reported. However, under ball milling conditions, IBX works efficiently with various organic functionalities at ambient temperature under solvent free conditions. Also, the waste IBA (2-iodosobenzoic acid) produced from the reactions was in situ oxidized to IBX in the following step using oxone and thus reused for multiple cycles by conserving its efficiency (only ∼6% loss after 15 cycles). This work describes an overview of a highly economical synthetic methodology which overcomes the problems of using IBX, efficiently in gram scale and in a non-explosive way.
Introduction
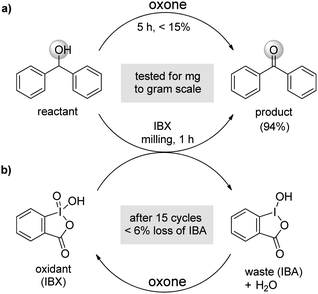
With rising public concern over alternative energy1 and global warming, it is important to trim down the usage of chemicals in routine chemical synthesis. In addition, eliminating waste and developing recyclable methodology is an essential aspect for doing reactions in a greener fashion.2 Similarly, metal free reagents are very popular in the pharmaceutical industry to avoid toxic metal contamination in drugs.3 2-Iodoxybenzoic acid (IBX) is a mild, environmental benign and easily available hypervalent iodine(V) reagent that has been preferred for metal free oxidative transformations in organic synthesis.4–7 Although journey of IBX started in 1893 by Hartmann and Meyer8 but its industrial/laboratory scale use was restricted due to explosiveness at higher temperature9 and poor solubility in common organic solvents except DMSO.10 In solvent DMSO, the large scale (gram quantities) syntheses using IBX have practical difficulties in isolation and purification. The limited solubility and explosive nature of IBX has encouraged many investigators to develop modified-IBX. After the first report on modified IBX i.e., Dess–Martin Periodinane11 in 1983 (DMP, Fig. 1), several modified IBX12–24 and solid-supported IBX25 are reported in literature to overcome the constraint of using IBX as utile oxidant. Recently, several research groups like Nicolaou,9,26–29 Zhdankin,30 Ishihara,5,31,32 Moorthy,23,24 Wirth21,33–35 and others7,36,37 have thoroughly explored the potential use of hypervalent iodine reagent (DMP, IBX etc.) in organic synthesis.30,37–41 Nowadays, the hypervalent iodine reagents are gaining popularity in organic syntheses because of their application in electron transfer chemistry.42,43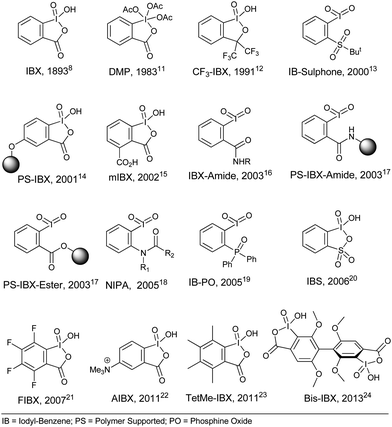 | ||
| Fig. 1 Selected and updated information on modified-IBX from 1893 and the abbreviated names are from the references shown as superscript. | ||
On the contrary, these modified IBXs are most often complicated due to involvement of tedious synthetic procedure in non-economical way.24 Therefore the modification approach has not been converged and is still in demand for better methods via recyclable methodology.44 We envisioned that ball milling methodology45,46 may possibly be used to make IBX compatible with various organic functionalities without introducing any modification. Herein we are investigating the potential use of IBX47 under solvent free, milling condition at room temperature to the following reactions: mainly oxidation of primary/secondary alcohols to corresponding carbonyl compounds,32 amine to imine,9 conversion of olefins to α-bromo/iodoketones,48 sulfide to sulfoxide,49 dithianes deprotection9 and synthesis of benzimidazoles from primary alcohols50 etc. (vide infra). Advantageously, this methodology has been established for long-range working window e.g., 10 mg to 2.5 grams.51 Also, the waste 2-iodosobenzoic acid (IBA) was recyclable to multiple cycles by in situ oxidative regeneration of IBX.52 This methodology does not require any aqueous workup, avoids chromatographic purification and found to be highly cost effective to be successfully used in pharmaceutical/chemical industry.
In Fig. 1, selective examples of modified IBXs are shown. Now, we are highlighting few shortcomings of these modified systems. The Dess–Martin Periodinane is soluble in common organic solvents but requires anhydrous condition for storing the reagent.11 Besides, CF3–IBX12 undergoes rapid ligand exchange with water–acetonitrile solution, water soluble mIBX15 is non-reactive towards non-allylic/benzylic alcohols, and FIBX21 have influence on acid sensitive reactions. In addition, AIBX,22 TetMe-IBX23 and bis-IBX24 are synthetically challenging. Polymer supported IBX25 which can work under heterogenous media, easily separable by filtration, are generally expensive.14 IBX is also known to works in ionic liquids,53 in aqueous medium with β-cyclodextrin (β-CD) through formation of host–guest complexes54 and in solid state at elevated temperature (70–90 °C) which associated with explosiveness, uncontrollable over-oxidation of primary alcohols to acids.55
Results and discussion
We have tested our methodology on oxidation of alcohols to carbonyls5 and the results are depicted in Fig. 2. The oxidized products of primary, secondary and aliphatic alcohols were obtained in very good to excellent yield in relatively smaller time. Now we are comparing our method with few literature known systems. For example, 2e (Fig. 2) was prepared in 45 min using IBX under ball-milling than 8 h using IBX–CH3CN–AcOH (traditional) method.5 The synthesis of 2w (Fig. 2) using bis-IBX (Fig. 1) is reported for 11.5 h in MeCN–H2O24 rather than 1 h under ball-milling. In continuation, the supramolecular system like cucurbit[8]uril catalyzed oxidation of alkyl alcohols 2p to corresponding aldehyde with IBX in aqueous solvent is known to be <5%,56 however our methods resulted the same in 77% yield. Thus, primary alcohols are efficiently converted to the corresponding aldehydes and no over oxidation to acids were found. The results shown in Fig. 2 are obtained by ball-milling process and this clearly establishes that the disadvantages associated with poor solubility of IBX are now overcome. | ||
| Fig. 2 Oxidation of alcohols to carbonyls; also shown compounds number, isolated yields and reaction times. | ||
The efficiency and convenience of this methodology on alcohol oxidations have also encouraged us to further explore the scope to verify other IBX-mediated literature reported reaction systems. As described in Fig. 3, these literature known examples are amine to imine,9 conversion of olefins to α-bromo/iodoketones,48 sulfide to sulfoxide,49 dithianes deprotection9 and synthesis of benzimidazoles from primary alcohols50 etc.
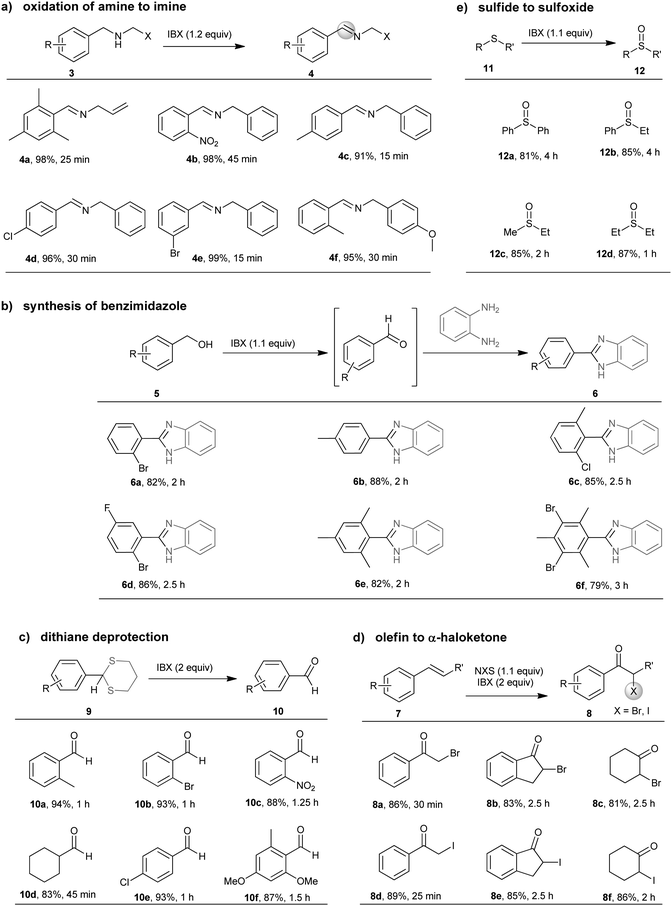
Fig. 4 represents the efficiency and recycling ability of this methodology. Waste IBA i.e., the reduced product of IBX after reaction, was isolated by paper filtration and subsequently in situ oxidized to IBX52 with inexpensive oxone in following recycling step. Importantly, towards waste management via recycling, in oxidation of benzhydrol to benzophenone (yield 94%, 1 h), after 15 cycles no significant loss (∼6%) of IBA was observed. However, oxone in absence of IBA could not efficiently oxidize benzhydrol (conversion < 15%, 5 h) under milling condition. On the other hand, oxidized products were isolated after filtration and purified. Most of the compounds were found to be sufficiently pure and did require chromatographic purification (HPLC analysis) to be used for synthetic applications.
The reaction shown in Fig. 5 is truly advantageous in terms of cost effectiveness. Per gram synthesis of 2-bromo benzaldehyde from (2-bromophenyl)methanol using IBX, in a single step using ball-milling, we could save nearly 48% of the estimated cost (Fig. 5, electricity, manpower costs are excluded). However in recycling the same reaction with IBA and oxone, additional 31% could be saved after one recycle (Fig. 5).
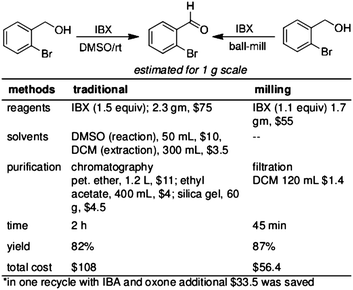 | ||
| Fig. 5 Comparative statement on economic benefit may be obtained from our methodology over traditional one. Using 25 mL of ZrO2 jar, 2.5 g of (2-bromophenyl)methanol was successfully oxidized. | ||
In this work we have demonstrated an outline of a highly economical synthetic methodology which overcomes the problem of using IBX for large scale synthesis. The solubility issue related to using IBX is also addressed carefully. Still, this method has certain limitations e.g., doing any reactions with aniline derivatives and for oxidation of toluenes to benzaldehydes. In both the cases detonation of the reaction mixture was observed. In addition, imines and benzimidazoles have tendency to get decomposed at room temperature and open atmosphere. Similarly, during HPLC analysis (ESI†) in polar protic solvent decomposition was also observed for imines and benzimidazoles. However, compounds were stable for longer time (weeks) under inert atmosphere and at low temperature (−20 °C). We have observed loss of materials while working with volatile compounds like sulfoxides (Fig. 3e) and α-haloketones (Fig. 3d) and therefore isolated yields were found to be lesser than expected.
Conclusions
We anticipate that a broad range of substrates is compatible with this operationally simple organo-oxidant IBX which works under solvent free condition, at room temperature, non-explosive way and also avoids aqueous workup. This methodology adapts in to milder reaction condition and is highly economical, time saving and reducing waste through smarter recycling. Therefore, this methodology may serve as an important addition not only to the synthetic field but also to industries. Thus, after 120 years of its discovery IBX may quench the thrust of modified-IBX and has a huge potential in solving a long-standing problem of organic chemistry. Our study will certainly be of interest to other researchers working not only on the development of hypervalent iodine mediated oxidation methodologies but also to chemists looking for better methodologies under the research area of organic mechanochemistry.57Experimental
Procedure for (2-bromophenyl)methanol oxidation under ball-milling
(2-Bromophenyl)methanol (100 mg, 0.53 mmol), IBX (162 mg, 0.58 mmol) and one grinding ball (15 mm diameter, ZrO2) were placed in a ball milling 10 mL ZrO2 jar. The progress of the reaction under milling condition was monitored by thin layer chromatography (TLC) and 1H NMR spectroscopy.58 After completion of the reaction, the reaction mixture was then transferred into 30 mL of dichloromethane (DCM), followed by product was isolated by paper filtration and waste IBA as precipitate. The resulting filtrate were concentrated in vacuum to isolate 85 mg (yield 87%) of 2-bromobenzaldehyde (2e) as colourless liquid.Optimization of gram scale reaction under ball-milling with (2-bromophenyl)methanol
(2-Bromophenyl)methanol (2.5 g, 13.4 mmol) and IBX (4.12 g, 14.7 mmol) were transferred to a milling 25 mL ZrO2 jar containing one 15 mm diameter ZrO2 grinding ball. After completion of reaction at 1 h, the product 2-bromobenzaldehyde isolated by following the procedure as presented above (2.10 g, yield: 85%).Data for unknown compounds
Acknowledgements
We are thankful to DST (New Delhi, India; Grant no. INT/FINLAND/P-06 and SR/S1/IC-59/2010) for financial support. TKA and SM are thankful to UGC and CSIR (India), respectively for fellowship. We are also thankful to Dr C. Giri for his help during manuscript preparation and IMMT Bhubaneswar for elemental analysis.Notes and references
- R. B. N. Baig and R. S. Varma, Chem. Soc. Rev., 2012, 41, 1559–1584 RSC.
- Nonappa, K. Ahonen, M. Lahtinen and E. Kolehmainen, Green Chem., 2011, 13, 1203–1209 RSC.
- M. Valko, H. Morris and M. T. D. Cronin, Curr. Med. Chem., 2005, 12, 1161–1208 CrossRef CAS.
- T. Dohi and Y. Kita, Chem. Commun., 2009, 2073–2085 RSC.
- M. Uyanik and K. Ishihara, Chem. Commun., 2009, 2086–2099 RSC.
- V. V. Zhdankin, J. Org. Chem., 2011, 76, 1185–1197 CrossRef CAS PubMed.
- L. F. Silva, Jr and B. Olofsson, Nat. Prod. Rep., 2011, 28, 1722–1754 RSC.
- C. Hartmann and V. Meyer, Ber. Dtsch. Chem. Ges., 1893, 26, 1727–1732 CrossRef.
- K. C. Nicolaou, C. J. N. Mathison and T. Montagnon, J. Am. Chem. Soc., 2004, 126, 5192–5201 CrossRef CAS PubMed.
- A. Duschek and S. F. Kirsch, Angew. Chem., Int. Ed., 2011, 50, 1524–1552 CrossRef CAS PubMed.
- D. B. Dess and J. C. Martin, J. Org. Chem., 1983, 48, 4155–4156 CrossRef CAS.
- D. B. Dess and J. C. Martin, J. Am. Chem. Soc., 1991, 113, 7277–7287 CrossRef CAS.
- D. Macikenas, E. Skrzypczak-Jankun and J. D. Protasiewicz, Angew. Chem., Int. Ed., 2000, 39, 2007–2010 CrossRef CAS.
- G. Sorg, A. Mengei, G. Jung and J. Rademann, Angew. Chem., Int. Ed., 2001, 40, 4395–4397 CrossRef CAS.
- A. P. Thottumkara and T. K. Vinod, Tetrahedron Lett., 2002, 43, 569–572 CrossRef CAS.
- V. V. Zhdankin, A. Y. Koposov, B. C. Netzel, N. V. Yashin, B. P. Rempel, M. J. Ferguson and R. R. Tykwinski, Angew. Chem., Int. Ed., 2003, 42, 2194–2196 CrossRef CAS PubMed.
- W.-J. Chung, D.-K. Kim and Y.-S. Lee, Tetrahedron Lett., 2003, 44, 9251–9254 CrossRef CAS PubMed.
- U. Ladziata, A. Y. Koposov, K. Y. Lo, J. Willging, V. N. Nemykin and V. V. Zhdankin, Angew. Chem., Int. Ed., 2005, 44, 7127–7131 CrossRef CAS PubMed.
- B. V. Meprathu, M. W. Justik and J. D. Protasiewicz, Tetrahedron Lett., 2005, 46, 5187–5190 CrossRef CAS PubMed.
- A. Y. Koposov, D. N. Litvinov, V. V. Zhdankin, M. J. Ferguson, R. McDonald and R. R. Tykwinski, Eur. J. Org. Chem., 2006, 4791–4795 CrossRef CAS.
- R. D. Richardson, J. M. Zayed, S. Altermann, D. Smith and T. Wirth, Angew. Chem., Int. Ed., 2007, 46, 6529–6532 CrossRef CAS PubMed.
- L.-Q. Cui, Z.-L. Dong, K. Liu and C. Zhang, Org. Lett., 2011, 13, 6488–6491 CrossRef CAS PubMed.
- J. N. Moorthy, K. Senapati, K. N. Parida, S. Jhulki, K. Sooraj and N. N. Nair, J. Org. Chem., 2011, 76, 9593–9601 CrossRef CAS PubMed.
- S. Seth, S. Jhulki and J. N. Moorthy, Eur. J. Org. Chem., 2013, 2445–2452 CrossRef CAS.
- M. Mülbaier and A. Giannis, Angew. Chem., Int. Ed., 2001, 40, 4393–4394 CrossRef.
- K. C. Nicolaou, K. Sugita, P. S. Baran and Y. L. Zhong, J. Am. Chem. Soc., 2002, 124, 2221–2232 CrossRef CAS PubMed.
- K. C. Nicolaou, T. Montagnon, P. S. Baran and Y. L. Zhong, J. Am. Chem. Soc., 2002, 124, 2245–2258 CrossRef CAS PubMed.
- K. C. Nicolaou, P. S. Baran, Y. L. Zhong and K. Sugita, J. Am. Chem. Soc., 2002, 124, 2212–2220 CrossRef CAS PubMed.
- K. C. Nicolaou, P. S. Baran, Y. L. Zhong, S. Barluenga, K. W. Hunt, R. Kranich and J. A. Vega, J. Am. Chem. Soc., 2002, 124, 2233–2244 CrossRef CAS PubMed.
- M. S. Yusubov, D. Y. Svitich, A. Yoshimura, V. N. Nemykin and V. V. Zhdankin, Chem. Commun., 2013, 49, 11269–11271 RSC.
- M. Uyanik, D. Suzuki, T. Yasui and K. Ishihara, Angew. Chem., Int. Ed. Engl., 2011, 50, 5331–5334 CrossRef CAS PubMed.
- M. Uyanik, M. Akakura and K. Ishihara, J. Am. Chem. Soc., 2009, 131, 251–262 CrossRef CAS PubMed.
- T. Wirth, Angew. Chem., Int. Ed., 2001, 40, 2812–2814 CrossRef CAS.
- R. D. Richardson and T. Wirth, Angew. Chem., Int. Ed., 2006, 45, 4402–4404 CrossRef CAS PubMed.
- F. V. Singh and T. Wirth, Org. Lett., 2011, 13, 6504–6507 CrossRef CAS PubMed.
- H. Liang and M. A. Ciufolini, Angew. Chem., Int. Ed., 2011, 50, 11849–11851 CrossRef CAS PubMed.
- V. Satam, A. Harad, R. Rajule and H. Pati, Tetrahedron, 2010, 66, 7659–7706 CrossRef CAS PubMed.
- G. Wang, Q.-Y. Yu, J. Wang, S. Wang, S.-Y. Chen and X.-Q. Yu, RSC Adv., 2013, 3, 21306–21310 RSC.
- G. Majji, S. K. Rout, S. Guin, A. Gogoi and B. K. Patel, RSC Adv., 2014, 4, 5357–5362 RSC.
- M. Fujita, K. Mori, M. Shimogaki and T. Sugimura, RSC Adv., 2013, 3, 17717–17725 RSC.
- T. Dohi and Y. Kita, Chem. Commun., 2009, 2073–2085 RSC.
- T. Dohi, M. Ito, K. Morimoto, M. Iwata and Y. Kita, Angew. Chem., Int. Ed., 2008, 47, 1301–1304 CrossRef CAS PubMed.
- M. Ito, H. Kubo, I. Itani, K. Morimoto, T. Dohi and Y. Kita, J. Am. Chem. Soc., 2013, 135, 14078–14081 CrossRef CAS PubMed.
- D. Hirose, T. Taniguchi and H. Ishibashi, Angew. Chem., Int. Ed., 2013, 52, 4613–4617 CrossRef CAS PubMed.
- A. Stolle, T. Szuppa, S. E. S. Leonhardt and B. Ondruschka, Chem. Soc. Rev., 2011, 40, 2317–2329 RSC.
- G.-W. Wang, Chem. Soc. Rev., 2013, 42, 7668–7700 RSC.
- M. Frigerio, M. Santagostino and S. Sputore, J. Org. Chem., 1999, 64, 4537–4538 CrossRef CAS.
- J. N. Moorthy, K. Senapati and N. Singhal, Tetrahedron Lett., 2009, 50, 2493–2496 CrossRef CAS PubMed.
- V. G. Shukla, P. D. Salgaonkar and K. G. Akamanchi, J. Org. Chem., 2003, 68, 5422–5425 CrossRef CAS PubMed.
- J. N. Moorthy and I. Neogi, Tetrahedron Lett., 2011, 52, 3868–3871 CrossRef CAS PubMed.
- Using 25 mL of milling jar we could successfully perform the reactions on 2.5 gram scale and scaled down to 10 mg with a smaller jar (10 mL). In all the cases one ball with 15 mm diameter was used and also followed recommended maximum loading 1/3 volume of the jar capacity.
- A. P. Thottumkara, M. S. Bowsher and T. K. Vinod, Org. Lett., 2005, 7, 2933–2936 CrossRef CAS PubMed.
- Z. Liu, Z.-C. Chen and Q.-G. Zheng, Org. Lett., 2003, 5, 3321–3323 CrossRef CAS PubMed.
- K. Surendra, N. S. Krishnaveni, M. A. Reddy, Y. V. D. Nageswar and K. R. Rao, J. Org. Chem., 2003, 68, 2058–2059 CrossRef CAS PubMed.
- J. N. Moorthy, N. Singhal and P. Venkatakrishnan, Tetrahedron Lett., 2004, 45, 5419–5424 CrossRef PubMed.
- Y.-H. Wang, H. Cong, F.-F. Zhao, S.-F. Xue, Z. Tao, Q.-J. Zhu and G. Wei, Catal. Commun., 2011, 12, 1127–1130 CrossRef CAS PubMed.
- S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friscic, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413–447 RSC.
- The ball-milling apparatus was stopped and small portion of sample was collected from the jar to study either proton NMR/TLC (Thin Layer Chromatography). Followed by, the reaction was started and this operation time was excluded for reporting the reaction timing.
Footnote |
| † Electronic supplementary information (ESI) available: Supporting data and NMR spectra for all the literature known compounds. See DOI: 10.1039/c4ra00415a |
| This journal is © The Royal Society of Chemistry 2014 |