A cost effective and eco-friendly one-pot process for PC61BM synthesis under aerobic conditions†
Abstract
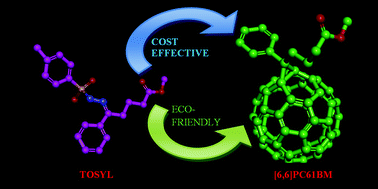
Here we demonstrate a cost effective and eco-friendly process for one-pot synthesis of PC61BM under aerobic conditions where, the key step of diazomethane intermediate preparation is modified. Instead of using pyridine and sodium methoxide under inert atmosphere, we used triethyl amine and dichloromethane under aerobic conditions. This process is envisaged as a green chemistry and will open channels for the large scale synthesis of PC61BM and its derivatives for solar cells applications without bothering for controlled environment conditions.

 Please wait while we load your content...
Please wait while we load your content...