Revisiting the principles of preparing aqueous quantum dots for biological applications: the effects of surface ligands on the physicochemical properties of quantum dots†
Butian Zhanga,
Rui Hua,
Yucheng Wanga,
Chengbin Yanga,
Xin Liub and
Ken-Tye Yong*a
aSchool of Electrical and Electronic Engineering, Nanyang Technological University, Singapore 639798, Singapore. E-mail: ktyong@ntu.edu.sg; Tel: +65-6790-5444
bDepartment of Chemical and Biological Engineering, University at Buffalo (SUNY), Buffalo, New York 14260, USA
First published on 5th March 2014
Abstract
Surface functionalization of quantum dots (QDs) is one of the most important aspects for the design and preparation of the desired QDs for specific biomedical applications. The surface ligands not only render the QDs water-dispersible, but also endow them with different functional groups for bioconjugation. More importantly, as the surface ligand layer on the QD surface is responsible for interacting with the biological environments, the type of surface ligand will greatly affect the response from the cells, such as the cellular uptake and cytotoxicity. In this paper, we investigate the effects of the surface ligand on the physicochemical properties of QDs and examine different QD formulations for possible biomedical applications. Seven types of QD formulations were prepared by anchoring the CdSe/CdS/ZnS QDs surface with either short-chain mercapto ligands (MPA, MSA, cysteine, AET) or PEG derivative ligands (mPEG-SH, CM-PEG-SH, NH2-PEG-SH). We then conducted a systematic study to evaluate the colloidal stability, photostability, cellular uptake and in vitro toxicity of the formulations. The colloidal stability was evaluated by the particle size change in water, acidic/neutral/alkaline buffer solutions and cell culture medium. Our results show that the carboxyl-terminated QDs have the best colloidal stability in water and alkaline solutions. PEG-capped QDs are more stable than short-chain ligand modified QDs in cell culture medium. For the photostability of different QD formulations under UV irradiation, we observed that the MPA-, MSA- and Cys-QDs had better photostability than that of the PEG modified QDs, whereas the AET-QD is the least stable one. Cellular uptake of QDs was evaluated using cell imaging and quantified by flow cytometry. The PEG chains and surface charge of QDs were found to play critical roles in the cellular uptake. Using RAW246.7 macrophage cells as the cellular uptake model, we discovered that the anionic QDs had a much higher uptake compared to the cationic QD formulations. In general, each set of prepared QD formulation with a specific type of surface ligand display certain strengths and limitations in different aspects of their physicochemical properties. Therefore, one should carefully consider and choose the type of QD formulation in the experiments thereby minimizing its impacts arising from their limitations.
Introduction
For the last decade, quantum dots (QDs) have shown great potential in biomedical applications.1–7 In comparison to conventional organic dyes, they possess comparable quantum yield (QY), excellent photostability, broad absorption spectra, narrow photoluminescence (PL) spectra and long fluorescence lifetimes.8–10 Moreover, their emission peak is size- and shape-dependent thereby allowing one to use them for multiplex fluorescence imaging in vitro and in vivo.11,12 These unique optical properties have made QDs promising probes for applications ranging from sensing to medicine.7,13–15 However, there remains a significant challenge to preserve the optical property of water-dispersible QD formulations with long shelf lives. Currently, many of the QD formulations are known to have short shelf lives from few hours to few days.3,16–18 As time progresses, some precipitation or aggregation of QDs will take place in aqueous phase or biological buffers. Such phenomena will adversely impact the optical property of QDs and may lead to skewing of data analysis when different quality of QDs is used in the experiments. Thus, there is an urgent need to further investigate and understand the root cause of the degradation of the optical property of QDs in biological mediums. In general, QDs are commonly synthesized in a reaction mixture containing organic solvents and surfactants.19,20 As such, the QDs prepared in this method are capped with hydrophobic ligands on their surface and they do not readily dispersed in aqueous phase and further surface modification steps are needed to make them water dispersible for biological applications.21–23 These surface modification steps not only made QDs water dispersible, but they also allow one to insert additional functionalities to the QDs formulations such as conjugating of biomolecules for targeting delivery and functionalization of MRI contrast agents for multimodal imaging.24,25 Thus, it is crucial for one to carefully design and optimize the surface modification steps for creating long shelf life of water dispersible QDs with comparable optical property as they are dispersed in organic phase for biomedical use.There are two approaches that have been commonly used for making water dispersible QDs. The first approach involves encapsulating QDs surface with biocompatible materials such as amphiphilic polymers26 and inorganic shells.27,28 This method offers the advantage of protecting the QD core from degradation in the biological environment. It is worth noting that this method will cause the overall QDs size to increase and may impact the intracellular mobility of the QDs if additional surface modification steps are not applied.3,29 The second method involves ligand exchange process. In the ligand exchange process, hydrophobic surfactants-functionalized QDs dispersion is mixed with excessive amount of heterobifunctional surfactants such as mercaptoacetic acid, mercaptosuccinic acid, mercaptopropionic acid, aminoethanethiol, allowing functional groups (e.g. thiol or amine group) attaching on the QD surface, and the other functional groups (e.g. carboxyl or amino group) extending out from the QDs surface to favorably interact with water.30–32 During the ligand exchange process, the hydrophobic moieties attached to the QD surface are displaced by the heterobifunctional surfactants through a mass driven process and the bifunctional ligands depositing to the QD surface will provide them with water dispersibility. In some occasions, monofunctional surfactants (e.g. thiolated PEG molecules and amino PEG molecules) are used to functionalize the QDs surface for specific applications such as long term in vivo imaging. The variation of ligands used in functionalizing the QDs surface will greatly influence the physical, colloidal and optical property of the QDs formulation. For instance, the overall QDs hydrodynamic size is mainly determined by the structure and length of the ligands use during the ligand exchange process.33 The surface charge density of QDs is affected by the functional groups and the grafting density of the ligands anchored on the QDs surface.34 The colloidal stability of the QDs is governed by the polarity of functional groups present in the ligands. Generally, solubility is controlled by intermolecular forces at the molecular level and the polarity level of the molecules plays an important role for dissolving in polar solvents such as water and alcohols. This is why QDs functionalized with less polar ligands like mercaptoundecanoic acid or mercaptohexadecanoic acid do not disperse well in water but they do disperse to a good extent in most organic solvents.35 Also, it is reported that the degradation of QDs is effected by the types of surface ligands functionalized on their surface.36 In this work, we perform a comprehensive study on investigating the effects of surface ligands on the overall physical, colloidal and optical property of QDs and systematically evaluating the suitability of each type of ligand-functionalized QDs formulations for biological applications. Specifically, seven common types of surface ligands with different structures, lengths and functional groups (mercaptopropionic acid, mercaptosuccinic acid, cysteine, aminoethanethiol, methoxy-PEG-thiol, carboxymethyl-PEG-thiol and amine-PEG-thiol molecules) were chosen to prepare water dispersible CdSe/CdS/ZnS QDs where these QDs were originally capped with hydrophobic surfactants such as trioctylphosphine (TOP) and trioctylphosphine oxide (TOPO). The colloidal stability, photostability, cellular uptake and cytotoxicity of the prepared QD formulations were systematically studied and compared. Some important guidelines are highlighted to prepare the best quality of QDs aqueous dispersion for imaging applications. Our study has provided useful information on understanding the impacts of surface ligands in designing specific type of QDs for different intended biomedical use.
Materials and experimental methods
Materials
3-Mercaptopropionic acid (MPA), mercaptosuccinic acid (MSA), DL-cysteine (Cys, 97%), cysteamine hydrochloride (2-aminoethanethiol hydrochloride, AET·HCl, ≥98%), chloroform, methanol and ammonium hydroxide solution (NH4OH, 28.0–30.0% NH3 basis) were purchased from Sigma-Aldrich. Methoxy-PEG-thiol (mPEG-SH, Mw 5000), carboxymethyl-PEG-thiol (CM-PEG-SH, Mw 5000) and amine-PEG-thiol (NH2-PEG-SH, Mw 5000) were obtained from Laysan Bio Inc. n-Hexane (95%) was purchased from Tedia. CertiPUR buffer solutions (pH 4.00/7.00/10.00, 20 °C) were products of Merck. Phosphate buffered saline (PBS) 7.2 was purchased from Gibco. Milli-Q 18.2 MΩ cm deionized (DI) water was used through the experiment. All chemicals were used as received without further purification. CdSe/CdS/ZnS QDs coated with TOP/TOPO were prepared using hot colloidal synthesis method and they were dissolved in chloroform and washed with ethanol before use.Preparing water-dispersible QDs using ligand exchange process
To prepare water-dispersible QDs, seven kinds of thiolated ligand molecules were used for the QDs phase transfer process. The typical methods for the QDs phase transfer process are replacing the TOP/TOPO capping layer on QDs by short-chain mercapto ligands (e.g. MPA, MSA, Cys and AET) and thiolated PEG derivatives (e.g. mPEG-SH, CM-PEG-SH and NH2-PEG-SH). In our study, we have optimized the reaction parameters in the phase transfer process for obtaining water-dispersible QDs with the highest quality of enhanced PL intensity and biocompatibility. In the case of modifying the QDs surface with short-chain thiol ligands, MPA (4 μl) or MSA (33 mg) dissolved in 1 ml of chloroform or Cys (2 mg) dissolved in 1 ml of methanol was mixed with 1 mg of TOP/TOPO-capped CdSe/CdS/ZnS QDs dispersed in 5 ml of chloroform and the mixture were stirred for 5 min. After 5 min, 1 ml of NH4OH solution with optimal concentration (3% for MPA, MSA and 20% for Cys respectively) was added into the reaction mixture and the biphasic solution system was continued to stir for another 5 h. After the stirring, majority of QDs were transferred to aqueous phase and they were then collected and washed by centrifugation with ethanol, and subsequently redispersed and stocked in 1 ml of DI water. To modify the QDs surface with AET surfactants, 100 μl of AET·HCl solution in methanol (5 mg ml−1) was added dropwise into 5 ml of QDs chloroform solution (0.2 mg ml−1), followed by vigorous stirring. At this stage, flocculation was formed in the system and the system was then dissolved by DI water with gentle shaking. After 10 min centrifugation (6000 rpm) to remove agglomerates, the AET capped QDs was collected by 100 kDa filter with centrifugation and redispersed in DI water for future use. To prepare PEGylated QDs, mPEG-SH (8 mg), CM-PEG-SH (5 mg) or NH2-PEG-SH (10 mg) dissolved in 2 ml of chloroform was mixed with 100 μl of QD chloroform solution (10 mg ml−1) and the mixture was stirred overnight. The QDs were then collected by adding hexane to the mixture. Before dispersing QDs to the aqueous phase, they were washed with the mixture of ethanol and hexane (v/v ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) and centrifuged at a speed of 6000 rpm for 10 min.
5) and centrifuged at a speed of 6000 rpm for 10 min.
QDs characterization
Fourier transform infrared (FT-IR) spectra were measured by a Shimadzu FT-IR spectrometer. Transmission electron microscopy (TEM) images were obtained using a JEOL JEM 2010 microscope. UV-vis absorption spectra of QDs dispersion were recorded using a Shimadzu UV-2450 spectrometer. The photoluminescence emission spectra of QDs dispersion were collected on a Fluorolog-3 Fluorometer (HORIBA Jobin Yvon, Edison, NJ USA). Zeta potential of the prepared QDs formulations was obtained by a ZetaPALS zeta potential analyzer (Brookhaven Instruments, NY USA). The QDs photostability study was performed by using G10T8 UV lamp (watts: 10 W, spectral output: 254 nm) for irradiation.Investigating the stability of QDs in water, buffer solutions and DMEM medium
To evaluate the colloidal stability of the prepared QD formulations, the hydrodynamic diameters of the prepared QDs dispersed in DI water, CertiPUR buffer solutions (pH = 4.00, 7.00 and 10.00, at 20 °C) and Dulbecco's Modified Eagle's Medium (DMEM, Gibco) were monitored by using dynamic light scattering (DLS) technique. A 90Plus particle size analyzer (Brookhaven Instruments, NY USA) was used in this study.Cell uptake of QDs study
RAW246.7 macrophage cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS, Hyclone), penicillin (100 μg ml−1, Gibco) and streptomycin (100 μg ml−1, Gibco) in a humidified environment (37 °C, 5% CO2). Before treating the cells with QDs, cells were seeded onto cover glasses in a 12-well plate with DMEM medium. The prepared QD formulations were then diluted to the same PL intensity for cellular uptake study. Next, the cells were treated with different QD formulations for 4 h. After 4 h of incubation, the treated cells were washed with PBS buffer for three times and fixed using 4% of formaldehyde solution. The nuclei were stained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI, Sigma) for imaging analysis. Nikon Eclipse Ti inverted Microscope with a 60× oil-immersion lens was used for cell imaging study. Flow cytometry study was performed using a FACSCalibur flow cytometer system (Becton Dickinson, Mississauga, CA).Cell viability study
Cell viability was performed by using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, Sigma) assay. Cells seeded in 96-well plates were incubated with QD formulations with different concentrations (sample wells) or same amount of PBS (control wells) for 24 h. It should be noted that the concentrations of the different QD formulations were determined based on the mass of the TOP/TOPO QDs before ligand exchange. After that, 18 μl of MTT (5 mg ml−1) solution was added to each well and the cells were incubated for another 4 h at 37 °C with 5% CO2. Then the solution in the wells was decanted and the purple precipitate was solubilized by adding 150 μl of dimethyl sulfoxide (DMSO, Sigma) to the sample wells. The absorbance of the solution in the sample wells was measured with a microplate reader (Bio-Rad) at a wavelength of 490 nm. The cell viability was obtained by normalizing the absorbance of the sample wells against that of the control wells.siRNA delivery by AET capped QDs
siRNAFAM was mixed with different amount of AET-QDs formulations. After 20 min incubation at room temperature, the mixtures were loaded on a 1.2% agarose gel and electro-phoresed at 100 V for 15 min. RAW246.7 macrophages were incubated with the QD-siRNAFAM complexes for 3 h, after that fluorescent imaging was taken.Results and discussion
In this work, we have prepared CdSe/CdS/ZnS QDs based on our previous reported method.37 The synthesized QDs are dispersed in organic solvent and capped by TOP/TOPO surfactants. Seven different surfactants, namely, MPA (mercaptopropionic acid), MSA (mercaptosuccinic acid), Cys (DL-cysteine), AET (2-aminoethanethiol hydrochloride), mPEG (methoxy-PEG-thiol), CM-PEG (carboxymethyl-PEG-thiol) and NH2-PEG (amine-PEG-thiol) were used for preparing water dispersible QDs surface via ligand exchange process. FT-IR spectroscopy was firstly used to examine the exchange of the capping ligands on QDs. Fig. 1 shows the representative FT-IR spectra of MSA, mPEG and TOP/TOPO capped QDs. The broad bands around 3000–3600 cm−1 for all the samples may arise from the hydroxyl groups and H2O bound on the QDs surface.38,39 The characteristic bands of S–H stretch at 2565 cm−1 are present in MSA molecules. Fig. 1a shows that the S–H stretch band disappeared after the ligand exchange process, which indicates the successful bonding between the sulfur atom and the QD surface.40,41 Also, the broad P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bands around 1074 cm−1
O bands around 1074 cm−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42,43 for TOP/TOPO capped QDs almost disappeared after MSA bonding. These changes suggest that the original TOP/TOPO on QDs surface is effectively replaced by MSA ligands. For ligand exchange with mPEG, strong peak of –CH stretch at 2886 cm−1, C–O–C stretch at 1114 cm−1
42,43 for TOP/TOPO capped QDs almost disappeared after MSA bonding. These changes suggest that the original TOP/TOPO on QDs surface is effectively replaced by MSA ligands. For ligand exchange with mPEG, strong peak of –CH stretch at 2886 cm−1, C–O–C stretch at 1114 cm−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 and other characteristic bands of mPEG45 are observed in mPEG capped QDs, indicating the coating of mPEG on QD surface (Fig. 1b). However, as these characteristic bands overlap with the –CH vibrations and P
44 and other characteristic bands of mPEG45 are observed in mPEG capped QDs, indicating the coating of mPEG on QD surface (Fig. 1b). However, as these characteristic bands overlap with the –CH vibrations and P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch, we were not able to determine whether the TOPO are completely removed. Fig. 2a shows the absorption and photoluminescence (PL) spectra of the hydrophobic moieties-functionalized QDs and various surface ligands-modified QD formulations. Upon comparing to the TOP/TOPO-coated QDs chloroform dispersion, no significant changes were observed for the absorption and PL spectra of the prepared water-dispersible QD formulations. Fig. 2b shows the TEM image of the QDs capped with TOP/TOPO surfactants. The high resolution image shows that the QDs are highly crystalline and have an average diameter of 6 nm. After ligand exchange process, the hydrodynamic sizes of the QDs were measured using DLS technique. The result shows that AET-QDs (∼25 nm) display a larger hydrodynamic diameter than that of MPA-, MSA- and Cys-QDs (7–10 nm). From the TEM images, we can also observe some agglomerates after modifying the QDs surface with AET (Fig. 2c). This is probably because the QD formulations may form different extents of ionic combinations and similar observations have been reported by Hoshino et al.36 Fig. 3 shows the zeta potential values for all the prepared surface modified QD formulations. QDs modified with surfactants containing carboxyl group show negatively charged surface and the QDs functionalized with surfactant containing amino group are determined to be positively charged. It should be noted that, for Cys-capped QDs, the negative zeta potential value could be attributed to the domination of the carboxyl group dissociation rather than the amino group protonation under the pH of DI water (the isoelectric point of cysteine is pH = 5.02). In addition, we have observed that surface ligand possesses with only carboxyl or amino groups (e.g. MPA, MSA, AET) will result in a much higher absolute zeta potential values upon comparing them to long-chain ligands (CM-PEG, NH2-PEG). This could be resulted either from the higher ligand packing density or the smaller size of ligands that are capped on the QDs surface.29 The different ligand dimensions may cause a wide diversity in ligand packing density. In this paper, we employed a geometric model to calculate the ligand packing density by assuming the spherical nanocrystals and cone-shaped ligands (Fig. S1†).46,47 Based on the ligand dimensions from previous experimental results,48–50 we estimated that the maximum possible packing density is ∼4524 per QD for short chain ligands and ∼355 per QD for PEG ligands. Considering the details such as the incomplete exchange of TOP/TOPO51 and the available anchor sites on QDs surface, the real situation could be more complicated. Nevertheless, this estimation still reveals the significant difference of the packing density between the short chain and PEG long chain ligands.
O stretch, we were not able to determine whether the TOPO are completely removed. Fig. 2a shows the absorption and photoluminescence (PL) spectra of the hydrophobic moieties-functionalized QDs and various surface ligands-modified QD formulations. Upon comparing to the TOP/TOPO-coated QDs chloroform dispersion, no significant changes were observed for the absorption and PL spectra of the prepared water-dispersible QD formulations. Fig. 2b shows the TEM image of the QDs capped with TOP/TOPO surfactants. The high resolution image shows that the QDs are highly crystalline and have an average diameter of 6 nm. After ligand exchange process, the hydrodynamic sizes of the QDs were measured using DLS technique. The result shows that AET-QDs (∼25 nm) display a larger hydrodynamic diameter than that of MPA-, MSA- and Cys-QDs (7–10 nm). From the TEM images, we can also observe some agglomerates after modifying the QDs surface with AET (Fig. 2c). This is probably because the QD formulations may form different extents of ionic combinations and similar observations have been reported by Hoshino et al.36 Fig. 3 shows the zeta potential values for all the prepared surface modified QD formulations. QDs modified with surfactants containing carboxyl group show negatively charged surface and the QDs functionalized with surfactant containing amino group are determined to be positively charged. It should be noted that, for Cys-capped QDs, the negative zeta potential value could be attributed to the domination of the carboxyl group dissociation rather than the amino group protonation under the pH of DI water (the isoelectric point of cysteine is pH = 5.02). In addition, we have observed that surface ligand possesses with only carboxyl or amino groups (e.g. MPA, MSA, AET) will result in a much higher absolute zeta potential values upon comparing them to long-chain ligands (CM-PEG, NH2-PEG). This could be resulted either from the higher ligand packing density or the smaller size of ligands that are capped on the QDs surface.29 The different ligand dimensions may cause a wide diversity in ligand packing density. In this paper, we employed a geometric model to calculate the ligand packing density by assuming the spherical nanocrystals and cone-shaped ligands (Fig. S1†).46,47 Based on the ligand dimensions from previous experimental results,48–50 we estimated that the maximum possible packing density is ∼4524 per QD for short chain ligands and ∼355 per QD for PEG ligands. Considering the details such as the incomplete exchange of TOP/TOPO51 and the available anchor sites on QDs surface, the real situation could be more complicated. Nevertheless, this estimation still reveals the significant difference of the packing density between the short chain and PEG long chain ligands.
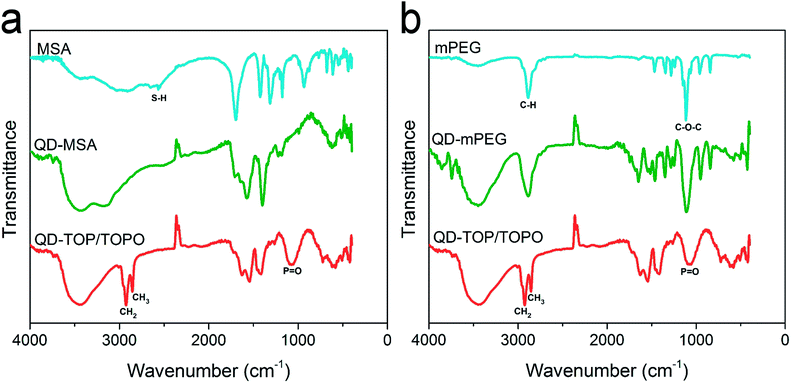 | ||
| Fig. 1 (a) FT-IR spectra of MSA, MSA capped QDs and TOP/TOPO capped QDs. (b) FT-IR spectra of mPEG, mPEG capped QDs and TOP/TOPO capped QDs. | ||
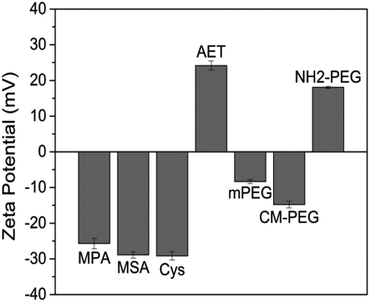 | ||
| Fig. 3 Zeta Potential measurements of QDs ligand exchanged with different surfactants: MPA, MSA, Cys, AET, mPEG, CM-PEG and NH2-PEG. | ||
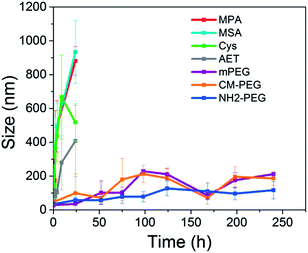
The colloidal stability of the prepared QD formulations in water was monitored by measuring their hydrodynamic size at various time points (see Fig. 4). All the prepared formulations here have different colloidal stability and shelf life ranging from period of days to weeks. The MPA- and CM-PEG-capped QDs are the most stable formulations as their hydrodynamic sizes kept almost unchanged over the course of more than 10 days. The MSA- and mPEG-QDs had a slight increase in the hydrodynamic size after aging the samples for one week while Cys- and NH2-PEG-QDs showed an obvious size increase due to aggregation of the nanoparticles. The Cys-capped QDs were discovered to be the formulation most prone to aggregation and this is probably due to the coexistence of carboxyl and amino groups on the QDs surface. In general, QDs terminated with carboxyl groups on their surface displayed a much better colloidal stability than those terminated with amino groups on the particle surface. Some reports have demonstrated that the aggregation is attributed to the desorption of the ligands from the QDs surface and this is due to the instability of dynamic nature of thiol–Zn interactions on the QDs surface and the ease of oxidation of the ligands in aqueous dispersions.3,31
 | ||
| Fig. 4 Time dependent hydrodynamic size monitoring of MPA-QDs, Cys-QDs, AET-QDs, mPEG-QDs, CM-PEG-QDs and NH2-PEG-QDs in DI water. | ||
To further examine the colloidal stability of QDs, their hydrodynamic sizes in different pH buffer solutions (pH = 4, 7, or 10, at 25 °C) were monitored using DLS technique. The colloidal stability of QDs functionalized with short-chain ligands is susceptible to change in different pH environment. Fig. 5a shows the relatively stable conditions of MPA-, Cys-, MSA-, and AET-QD formulations under different pH environment. Other formulations that were not included into the plots had very poor colloidal stability and formed large aggregates during the first two to six hours of stability test. The MPA and MSA functionalized QD formulations are relatively stable in alkaline environment due to the ionization of the carboxyl groups. The aggregation and precipitation of other QD formulations might have been resulted from the weakening of Coulombic interactions between particles when they are dispersed in electrolyte solution.52 The Coulombic interaction of the nanoparticles arises from the repulsive electrostatic force between individual nanoparticles sharing similar surface charge potentials. On the other hand, the QDs capped with PEG chains were much more colloidally stable in buffer solutions. This is due to the steric stabilization from the long PEG chains and the hydrogen bonding of PEG molecules in water.23 Fig. 5b–d suggested that the surface functional groups on the PEG molecules play an important role in determining the colloidal stability life span of the QDs formulation in solutions of varying pH. The CM-PEG-QDs are much more stable in pH 10 buffer solution than in pH 4 solution while a different trend was observed for NH2-PEG-QDs formulation. Fig. 5d shows that aggregation of particles was observed for NH2-PEG-QDs formulation dispersed in both pH 4 and 10 buffer solutions. This is not surprising since the ionization of the carboxyl/amino groups in alkaline/acidic environments might have manipulated particle surface charge whereby enhancing or weakening the electrostatic repulsion forces between particles. Aqueous solution at low pH has numerous hydrogen ions (H+) and ease the protonation of amino groups while suppress the dissociation of carboxyl groups. On the other hand, high pH solution is rich in hydroxide ions (OH−), which facilitates the dissociation of carboxyl groups but make it difficult for amino groups to acquire hydrogen to be ionized. Interestingly, we have observed that the PEG modified QDs do not favourably interact with water when they are placed in buffer with pH 7. All PEG modified QD formulations started to aggregate in pH 7 buffer solution after 25 to 100 hours. The hydrogen bonds forming between the PEG chains and the water molecules are the main factor that supports the colloidal stability of the QDs in electrolyte solution. However, the presence of various salt ions in the neutral buffer solution may have disrupted the formation of hydrogen bonding and thus leading the PEG-modified QDs to aggregate.53 For in vitro applications, the synthesized QDs are required to be mixed with the cell culture medium for imaging and sensing applications. In the cell culture medium, the solution contains amino acids, salts, glucose and vitamins that are essential for the cells growth and these ingredients play a key role in determining the colloidal stability of QDs.54
Fig. 6 shows the variation of hydrodynamic diameter of DMEM (Dulbecco's modified Eagle's medium) dispersion of different QD formulations against time. From Fig. 6, there is clear surfactant dependence for lengthening the life span of the colloidal stability of DMEM/QDs dispersion. For the short-chain ligand modified QDs, they aggregated in DMEM solution less than a day. The size distribution of the QD aggregates was observed to vary from one hundred to a few hundreds of nanometers. For the case of DMEM dispersion of PEG (mPEG, CM-PEG and NH2-PEG) modified QDs, they are relatively more stable than that of short-chain ligands modified QDs since their hydrodynamic diameter size varies less comparing to their initial size. We speculate that this may be due to the PEG modified QDs have better resistance to adsorption of surrounding biomolecules.55
 | ||
| Fig. 6 Time dependence of the hydrodynamic size of MPA-QDs, MSA-QDs, Cys-QDs, AET-QDs, mPEG-QDs, CM-PEG-QDs and NH2-PEG-QDs in DMEM medium. | ||
Photostability is an important parameter to be considered for selecting the most appropriate fluorescent probes for bioimaging applications. QDs with outstanding photostability are appropriate for applications with long-term excitation and requiring accurate quantitative analysis, such as real-time molecule tracking and high-resolution 3D reconstruction.56 Generally, the photostability of QDs are affected by oxygen exposure level,57 solvents,58 reducing agents (e.g. mercaptoethanol, dithiothreitol) in solvents,59 the core compositions and core/shell structure of QDs60–62 and capping surfactants.57,63–65 The evolution of the PL spectra of QDs under irradiation is a common way to examine their photostability. In some cases, UV irradiation may enhance the PL intensity due to photo-induced smoothing and passivation of QD surface.58 Different mechanisms were proposed to explain the smoothing or passivation process of the QDs surface. For example, most thiol ligands (e.g. MAA, MPA) were reported to passivate the surface defects using adsorbed water molecules, surfactant molecules and new-formed oxide layer.44 However, the thioglycolic acid (TGA) stabilized QDs formed an additional CdS shell on the surface through the utilization of the sulphur source from TGA ligand.66 On the other hand, the UV irradiation may also induce corrosion of the QD core58 and aggregation of QDs,67 which in turn result in PL degeneration and intensity decrease. In addition, the size of QDs would also change due to photo-corrosion (decrease in size) or coagulation/ripening (increase in size), and consequently causing blue- or red-shift in the PL spectra.63 In this study, the photoluminescence (PL) spectra of all the prepared QD formulations were monitored under continuous exposure to UV irradiation. Fig. 7a shows an example of PL spectra for MPA-QDs formulation after exposing them with UV radiation for different time period and an increase in the PL intensity is observed as radiation exposure time increases. To provide a better understanding on the photostability of the prepared QD formulations, the maximum peak of PL spectra for each formulation was plotted against irradiation time and this is showed in Fig. 7b. Except for the AET-QDs formulation, an enhancement in the PL intensity was observed for all the QD formulations in the first few hours of UV irradiation. The variation of PL intensity is due to the photoadsorption and photooxidation reactions occurred on the QDs-thiol interface during the exposure of the QDs formulations to the strong UV irradiation. The photoadsorption reaction will help to remove the surface defects on the QDs surface through an improvement surface passivation process where the coordination of thiols (from ligands) or water molecules with QD surface atoms is enhanced whereby increasing the PL intensity of QDs.58,68 In the beginning of the photo-induced oxidation process, a thin oxide layer is formed to passivate the QDs and increase their PL intensity. However, further oxidation leads to the photo-corrosion and aggregation of QDs, of which both are responsible for the PL degeneration.44,67 Fig. 7b also showed that MPA-, MSA- and Cys-QDs had a longer and larger PL enhancement process than mPEG-, CM-PEG- and NH2-PEG-QDs. We speculate that this may arise from the difference in the grafting density of the thiol ligands on QDs. Since the PEG ligands are relatively large, they might have a lower grafting density on QD surface (ESI†). As a result, the PEG ligands could be consumed by oxidation more quickly during the PL enhancement process. The unprotected QDs surfaces are prone to be corroded and form surface defects, which causes a faster decrease of the PL intensity. As for the AET-QD formulation, it only showed a relatively quick decrease in the PL intensity. This might result from the instability of AET ligand, which is easily oxidized to its disulphide state at room temperature.69 Therefore, without the insulation by sufficient AET ligands, the partially naked QDs are liable to form surface defects or aggregate and thus display a quick decrease in their PL intensity.
Nonspecific cell uptake of QDs is a common strategy used to evaluate the feasibility of using the prepared QDs for bioimaging applications. The cellular uptake rate of QDs is greatly affected by several key factors derived from the surface coatings of the QDs, such as the size, surface charge and functional groups.34,70–72 It is also cell-type specific and dose dependent.72,73 The internalization of nanoparticles by SK-BR-3 cells was observed to be most efficient within 25–50 nm size range.74 This is a general rule for most nonphagocytic cells whereas phagocytic cells preferentially ingest particles in the range between 2 and 3 μm.73 The surface charge affects the cellular uptake but this influence is also cell-type specific. Park et al. have shown that HeLa cells have a larger and more rapidly uptake of positively charged QDs than negatively charged QDs while there is almost no internalization for neutral QDs.75 Similar results were found by Tan et al. for HepG2 and NIH3T3 cells.72 In contrast to these nonphagocytic cells, most phagocytic cells have stronger interaction with negatively charged particles.73 To reduce the nonspecific cellular uptake, various strategies were proposed, such as using zwitterionic QDs (e.g. bovine serum albumin (BSA),76 penicillamine77), lysine cross-linked mercapto-acid QDs35 and hydroxylated QDs.78 Among these strategies, PEGylation of the QD surface is the most commonly employed method. The reduction in the cellular uptake was attributed to minimal functional groups from PEG chains34 and the steric repulsive barrier between PEG-modified QDs and cells.79–81 Different length and density of PEG chains also account for different cellular uptake rates.34,82,83 It should be noted that nonspecific binding amount of nanoparticles varies for each cell type.76,82 In this work, the cell uptake of QDs by RAW246.7 macrophages was carried out for all the synthesized QD formulations. Fig. 8 shows the differential interference contrast (DIC) and fluorescent images of RAW246.7 macrophages cells treated with MPA-, Cys-, AET-, mPEG-, CM-PEG- and NH2-PEG-QD formulations. Upon comparing to short-chain ligands modified QDs, minimal uptake of PEG-functionalized QDs was observed in the cells, which is consistent with the previous reports.34,72,84 A strong uptake of MPA- and Cys-QDs was observed after treating the cells with QDs for 4 hours. MSA-QDs formulation was found to exhibit a lower uptake when compared to the MPA- and Cys-QD formulations even though their zeta potential value and hydrodynamic size were almost the same. The preference of QDs uptake may be influenced by the different aggregation state of the nanoparticles. Commonly, most nonphagocytic cells take up cationic nanoparticles to a higher extent than anionic nanoparticles72 whereas phagocytic cells interact preferentially with anionic nanoparticles.73 In our case, we observed that anionic charged QD formulations such as MPA-, Cys-, MSA-, mPEG-, CM-PEG-QDs displayed a higher cell uptake by RAW246.7 macrophage cells in comparison to the cationic charged QDs such as AET- and NH2-PEG-QDs. Sakai et al. showed that, for PC12 cells, the carboxyl-group modified QDs were taken up more effectively than the amino-group modified QDs.85 Clift et al. reported that the carboxyl-group modified QDs were rapidly taken up by the J774.A1 macrophage cells based on the endocytic mechanism.84 Our result is consistent with their observation where similarly we have determined that carboxyl-terminated QDs formulation is having a higher uptake than that of the amino-terminated QDs. In addition, the cellular uptake has been quantified by flow cytometry analysis. The representative plots of the fluorescence intensity in RAW246.7 macrophages treated with or without QD formulations were given in Fig. 9. The results are consistent with the patterns obtained from the fluorescent imaging. In general, a relatively high cellular uptake of QDs functionalized with short-chain ligands were observed upon comparing to PEG modified QDs. The strongest fluorescent signals were found in the cells treated with MPA-QDs and Cys-QDs formulation.
The toxicity of QDs has been extensively studied for the last decade to understand their impact on the biological systems. Early studies revealed that the QDs attached with ligands such as MPA and AET were degraded in the biological environment due to the oxidation of the QDs and this caused the release of heavy metal ions (e.g. Cd2+, Pb2+) from the particle surface.86,87 Applying cross-linked molecules (e.g. BSA86) or multidentate polymers on the QDs surface (e.g. DHLA-PEG) may help to minimize the release of the toxic components, whereas using heavy-metal-free QDs (e.g. silicon QDs88) will lowered the QDs toxicity concern. Recently, Zheng et al. have examined the toxicity of a series of thiol-capped QDs.89 They have found that the negatively charged glutathione (GSH) coated QDs have the lowest toxicity while the positively charged polyethylenimine (PEI) coated QDs are the most cytotoxic to HaCaT cells among other formulations tested. In addition, incomplete purification90 of QDs sample may leave some unwashed impurities in the dispersion and induce the toxicological response.91 Besides from these factors, toxicity could also result from the intrinsic property of QDs, such as their size92 or reactive oxygen species (ROS) generated on the QDs surface when they are excited by light.93 It is worth noting that the physiological environment may also change the size, surface charge and agglomeration states of QDs and these changes will have a significant impact on the toxicological responses from the cells.94 Also, after QDs are introduced into the cell culture system or in the body, proteins will generally be absorbed onto the QD surface and such “complex” may result in changes of the protein activities whereby leading to certain dysfunctions in the biological parts in vitro and in vivo.95 All these impacts to the biological systems are directly related to the surface functionalization of the QDs. Thus, continuation and extensive studies are needed to further investigate and understand the underlying mechanisms of QDs toxicity in vitro and in vivo. In this study, the cytotoxicity of all the QD formulations was evaluated by MTT assays. Fig. 10 shows the cell viability of RAW246.7 macrophages cells after treating them with seven QD formulations for 24 hours. The cells treated with the MSA-QD formulation display the highest viability, followed by the NH2-PEG- and MPA-QD formulations. All the QD formulations maintained greater than 80% cell viability even at concentrations as high as 120 μg ml−1. It is worth mentioning that the concentrations used for cell imaging is much lower than the value of 120 μg ml−1 used in the MTT assays. This suggests that the prepared QD formulations have negligible toxicity for cell imaging thus could be functionalized with biomolecules and find wide applications for in vitro studies. As an example, in vitro cellular delivery of fluorescent FAM labeled siRNAs was demonstrated using AET-QDs as carriers. siRNAs are small interfering RNAs with a typical length of 20–25 base pairs. When delivered into the cytoplasm, the sequence-specific siRNAs initiate the RNA interference process and thus regulate the expression of a targeting gene for gene therapy.96,97 However, free siRNAs are fragile and negatively charged. They are liable to degradation in biological fluids or could not penetrate the cell membrane by themselves without a proper carrier.98,99 In this example, siRNAs were conjugated with the positively charged AET-QDs through electrostatic interaction (ESI, Fig. S2†) and were successfully delivered into cell cytoplasm (ESI, Fig. S3†).
 | ||
| Fig. 10 Relative cell viability of RAW246.7 macrophages treated with varying concentrations of MPA-QDs, MSA-QDs, Cys-QDs, AET-QDs, mPEG-QDs, CM-PEG-QDs and NH2-PEG-QDs for 24 h, n = 5. | ||
Conclusions
In summary, we have performed a detailed study in understanding the effects of surface ligands on the physical property, optical and colloidal stability, cellular uptake and in vitro cytotoxicity of QDs. Our result indicates that PEG modified QDs have a higher colloidal stability and they have lower uptake in the cells upon comparing to short-chain ligand modified QD formulations. However, PEG modified QDs displayed a relatively large hydrodynamic diameter size and they have poor photostability. For carboxyl-terminated QD formulations, these formulations possess an excellent colloidal stability in water and alkaline buffer solutions. MSA-QDs were revealed to be most stable in neutral and alkaline buffer solutions and also possess best photostability. Our study suggested that the preference of QDs cell uptake is strongly correlated with the surface charge density of QDs. We have found that the anionic QDs were having much higher cell uptake in comparison to the cationic QD formulations for RAW246.7 macrophage cells. Overall, the physicochemical property of QDs is sensitively influenced by the type of surface ligands used to modify the QDs surface. Thus, it is important to consider the length, surface charge, grafting density and functional groups of the surface ligands in designing the desirable QDs formulation for specific biological applications. It is worth noting that there are diverse QD ligands exchange protocols available in the literature and slight changes in the preparation parameters will result in different quality of QD aqueous dispersion. As such, it is imperative to follow the same set of protocol when preparing the QDs dispersion with specific type of surface ligands. In general, each set of prepared QD formulation with specific type of surface ligands will display some limitations in certain aspects of their physicochemical property and therefore we should carefully consider and choose the type of QDs required to be used in the experiments thereby minimizing their impacts arising from their limitation factors.Acknowledgements
The study was supported by the Start-up grant (M4080141.040) from Nanyang Technological University, Tier 1 Academic Research Funds (M4010360.040 RG29/10) from Singapore Ministry of Education and partially from the Singapore Ministry of Education under Tier 2 Research Grant MOE2010-T2-2-010 (4020020.040 ARC2/11).Notes and references
- F. Pinaud, S. Clarke, A. Sittner and M. Dahan, Nat. Methods, 2010, 7, 275–285 CrossRef CAS PubMed.
- A. M. Smith, H. W. Duan, A. M. Mohs and S. M. Nie, Adv. Drug Delivery Rev., 2008, 60, 1226–1240 CrossRef CAS PubMed.
- I. L. Medintz, H. T. Uyeda, E. R. Goldman and H. Mattoussi, Nat. Mater., 2005, 4, 435–446 CrossRef CAS PubMed.
- X. Michalet, F. F. Pinaud, L. A. Bentolila, J. M. Tsay, S. Doose, J. J. Li, G. Sundaresan, A. M. Wu, S. S. Gambhir and S. Weiss, Science, 2005, 307, 538–544 CrossRef CAS PubMed.
- M. Bruchez, M. Moronne, P. Gin, S. Weiss and A. P. Alivisatos, Science, 1998, 281, 2013–2016 CrossRef CAS.
- T. L. Doane and C. Burda, Chem. Soc. Rev., 2012, 41, 2885–2911 RSC.
- Y. C. Wang, R. Hu, G. M. Lin, I. Roy and K. T. Yong, ACS Appl. Mater. Interfaces, 2013, 5, 2786–2799 CAS.
- U. Resch-Genger, M. Grabolle, S. Cavaliere-Jaricot, R. Nitschke and T. Nann, Nat. Methods, 2008, 5, 763–775 CrossRef CAS PubMed.
- S. Jiang, K. Y. Win, S. H. Liu, C. P. Teng, Y. G. Zheng and M. Y. Han, Nanoscale, 2013, 5, 3127–3148 RSC.
- A. R. Clapp, I. L. Medintz and H. Mattoussi, ChemPhysChem, 2006, 7, 47–57 CrossRef CAS PubMed.
- W. C. W. Chan, D. J. Maxwell, X. H. Gao, R. E. Bailey, M. Y. Han and S. M. Nie, Curr. Opin. Biotechnol., 2002, 13, 40–46 CrossRef CAS.
- R. J. Byers and E. R. Hitchman, Prog. Histochem. Cytochem., 2011, 45, 201–237 CrossRef PubMed.
- M. V. Yezhelyev, L. F. Qi, R. M. O'Regan, S. Nie and X. H. Gao, J. Am. Chem. Soc., 2008, 130, 9006–9012 CrossRef CAS PubMed.
- C. J. Xu, L. Y. Mu, I. Roes, D. Miranda-Nieves, M. Nahrendorf, J. A. Ankrum, W. A. Zhao and J. M. Karp, Nanotechnology, 2011, 22, 494001 CrossRef PubMed.
- V. Biju, S. Mundayoor, R. V. Omkumar, A. Anas and M. Ishikawa, Biotechnol. Adv., 2010, 28, 199–213 CrossRef CAS PubMed.
- L. F. Qi and X. H. Gao, Expert Opin. Drug Delivery, 2008, 5, 263–267 CrossRef CAS PubMed.
- J. A. Kloepfer, R. E. Mielke and J. L. Nadeau, in Quantum Dots, Nanoparticles, and Nanoclusters, ed. D. L. Huffaker and P. Bhattacharya, 2004, vol. 5361, pp. 133–141 Search PubMed.
- B. K. Pong, B. L. Trout and J. Y. Lee, Langmuir, 2008, 24, 5270–5276 CrossRef CAS PubMed.
- V. Biju, T. Itoh and M. Ishikawa, Chem. Soc. Rev., 2010, 39, 3031–3056 RSC.
- V. Biju, T. Itoh, A. Anas, A. Sujith and M. Ishikawa, Anal. Bioanal. Chem., 2008, 391, 2469–2495 CrossRef CAS PubMed.
- R. A. Sperling and W. J. Parak, Philos. Trans. R. Soc., A, 2010, 368, 1333–1383 CrossRef CAS PubMed.
- N. Erathodiyil and J. Y. Ying, Acc. Chem. Res., 2011, 44, 925–935 CrossRef CAS PubMed.
- F. Zhang, E. Lees, F. Amin, P. R. Gil, F. Yang, P. Mulvaney and W. J. Parak, Small, 2011, 7, 3113–3127 CrossRef CAS PubMed.
- J. H. Lee, K. Lee, S. H. Moon, Y. Lee, T. G. Park and J. Cheon, Angew. Chem., Int. Ed., 2009, 48, 4174–4179 CrossRef CAS PubMed.
- R. Hu, Y. C. Wang, X. Liu, G. M. Lin, C. H. Tan, W. C. Law, I. Roy and K. T. Yong, RSC Adv., 2013, 3, 8495–8503 RSC.
- N. Tomczak, D. Janczewski, M. Y. Han and G. J. Vancso, Prog. Polym. Sci., 2009, 34, 393–430 CrossRef CAS PubMed.
- Y. Zhang, M. Wang, Y. G. Zheng, H. Tan, B. Y. W. Hsu, Z. C. Yang, S. Y. Wong, A. Y. C. Chang, M. Choolani, X. Li and J. Wang, Chem. Mater., 2013, 25, 2976–2985 CrossRef CAS.
- J. T. Song, S. J. Wu and Y. L. Zhao, Mater. Res. Bull., 2013, 48, 1530–1535 CrossRef CAS PubMed.
- W. Liu, M. Howarth, A. B. Greytak, Y. Zheng, D. G. Nocera, A. Y. Ting and M. G. Bawendi, J. Am. Chem. Soc., 2008, 130, 1274–1284 CrossRef CAS PubMed.
- S. B. Brichkin and E. V. Chernykh, High Energy Chem., 2011, 45, 1–12 CrossRef CAS.
- W. H. Liu, H. S. Choi, J. P. Zimmer, E. Tanaka, J. V. Frangioni and M. Bawendi, J. Am. Chem. Soc., 2007, 129, 14530–14531 CrossRef CAS PubMed.
- S. F. Wuister, I. Swart, F. van Driel, S. G. Hickey and C. D. Donega, Nano Lett., 2003, 3, 503–507 CrossRef CAS.
- A. M. Smith and S. Nie, J. Am. Chem. Soc., 2008, 130, 11278–11279 CrossRef CAS PubMed.
- T. A. Kelf, V. K. A. Sreenivasan, J. Sun, E. J. Kim, E. M. Goldys and A. V. Zvyagin, Nanotechnology, 2010, 21, 285105 CrossRef CAS PubMed.
- W. Jiang, S. Mardyani, H. Fischer and W. C. W. Chan, Chem. Mater., 2006, 18, 872–878 CrossRef CAS.
- A. Hoshino, K. Fujioka, T. Oku, M. Suga, Y. F. Sasaki, T. Ohta, M. Yasuhara, K. Suzuki and K. Yamamoto, Nano Lett., 2004, 4, 2163–2169 CrossRef CAS.
- L. Ye, K. T. Yong, L. W. Liu, I. Roy, R. Hu, J. Zhu, H. X. Cai, W. C. Law, J. W. Liu, K. Wang, J. Liu, Y. Q. Liu, Y. Z. Hu, X. H. Zhang, M. T. Swihart and P. N. Prasad, Nat. Nanotechnol., 2012, 7, 453–458 CrossRef CAS PubMed.
- M. Taibi, S. Ammar, N. Jouini, F. Fievet, P. Molinie and M. Drillon, J. Mater. Chem., 2002, 12, 3238–3244 RSC.
- J. W. Lee, W. C. Choi and J. D. Kim, CrystEngComm, 2010, 12, 3249–3254 RSC.
- S. S. L. Sobhana, M. V. Devi, T. P. Sastry and A. B. Mandal, J. Nanopart. Res., 2011, 13, 1747–1757 CrossRef.
- T. Y. Dong, W. T. Chen, C. W. Wang, C. P. Chen, C. N. Chen, M. C. Lin, J. M. Song, I. G. Chen and T. H. Kao, Phys. Chem. Chem. Phys., 2009, 11, 6269–6275 RSC.
- H. B. Li, C. P. Han and L. Zhang, J. Mater. Chem., 2008, 18, 4543–4548 RSC.
- R. C. Advincula, Dalton Trans., 2006, 2778–2784 RSC.
- C. Barrera, A. P. Herrera and C. Rinaldi, J. Colloid Interface Sci., 2009, 329, 107–113 CrossRef CAS PubMed.
- C. Y. Wang, L. L. Feng, H. Z. Yang, G. B. Xin, W. Li, J. Zheng, W. H. Tian and X. G. Li, Phys. Chem. Chem. Phys., 2012, 14, 13233–13238 RSC.
- J. E. B. Katari, V. L. Colvin and A. P. Alivisatos, J. Phys. Chem., 1994, 98, 4109–4117 CrossRef CAS.
- C. Bullen and P. Mulvaney, Langmuir, 2006, 22, 3007–3013 CrossRef CAS PubMed.
- G. Sui, J. Orbulescu, X. J. Ji, K. M. Gattas-Asfura, R. M. Leblanc and M. Micic, J. Cluster Sci., 2003, 14, 123–133 CrossRef CAS.
- W. P. Wuelfing, S. M. Gross, D. T. Miles and R. W. Murray, J. Am. Chem. Soc., 1998, 120, 12696–12697 CrossRef CAS.
- X. L. Huang, L. M. Bronstein, J. Retrum, C. Dufort, I. Tsvetkova, S. Aniagyei, B. Stein, G. Stucky, B. McKenna, N. Remmes, D. Baxter, C. C. Kao and B. Dragnea, Nano Lett., 2007, 7, 2407–2416 CrossRef CAS PubMed.
- M. Kuno, J. K. Lee, B. O. Dabbousi, F. V. Mikulec and M. G. Bawendi, J. Chem. Phys., 1997, 106, 9869–9882 CrossRef CAS PubMed.
- W. J. Parak, D. Gerion, T. Pellegrino, D. Zanchet, C. Micheel, S. C. Williams, R. Boudreau, M. A. Le Gros, C. A. Larabell and A. P. Alivisatos, Nanotechnology, 2003, 14, R15–R27 CrossRef CAS.
- V. V. Khutoryanskiy, G. A. Mun, Z. S. Nurkeeva and A. V. Dubolazov, Polym. Int., 2004, 53, 1382–1387 CrossRef CAS.
- I. Ojea-Jimenez, J. Piella, T. L. Nguyen, A. Bestetti, A. D. Ryan and V. Puntes, in Nanosafe 2012: International Conferences on Safe Production and Use of Nanomaterials, IOP, 2013, vol. 429 Search PubMed.
- A. J. Wang, J. J. Xu and H. Y. Chen, J. Chromatogr., A, 2007, 1147, 120–126 CrossRef CAS PubMed.
- P. Zrazhevskiy, M. Sena and X. H. Gao, Chem. Soc. Rev., 2010, 39, 4326–4354 RSC.
- S. Emin, A. Loukanov, M. Wakasa, S. Nakabayashi and Y. Kaneko, Chem. Lett., 2010, 39, 654–656 CrossRef CAS.
- C. Carrillo-Carrion, S. Cardenas, B. M. Simonet and M. Valcarcel, Chem. Commun., 2009, 5214–5226 RSC.
- E. A. Christensen, P. Kulatunga and B. C. Lagerholm, PLoS One, 2012, 7 Search PubMed.
- Y. He, H. T. Lu, L. M. Sai, Y. Y. Su, M. Hu, C. H. Fan, W. Huang and L. H. Wang, Adv. Mater., 2008, 20, 3416–3421 CrossRef CAS.
- F. Aldeek, C. Mustin, L. Balan, G. Medjahdi, T. Roques-Carmes, P. Arnoux and R. Schneider, Eur. J. Inorg. Chem., 2011, 794–801 CrossRef CAS.
- W.-S. Chae, T. D. T. Ung and Q. L. Nguyen, Adv. Nat. Sci.: Nanosci. Nanotechnol., 2013, 4, 045009 CrossRef.
- A. M. A. Morshed and T. H. Yoon, Bull. Korean Chem. Soc., 2008, 29, 249–251 CrossRef CAS.
- D. L. Nida, N. Nitin, W. W. Yu, V. L. Colvin and R. Richards-Kortum, Nanotechnology, 2008, 19 Search PubMed.
- Isnaeni, L. H. Jin and Y. H. Cho, J. Colloid Interface Sci., 2013, 395, 45–49 CrossRef CAS PubMed.
- H. Peng, L. J. Zhang, C. Soeller and J. Travas-Sejdic, J. Lumin., 2007, 127, 721–726 CrossRef CAS PubMed.
- J. Aldana, Y. A. Wang and X. G. Peng, J. Am. Chem. Soc., 2001, 123, 8844–8850 CrossRef CAS PubMed.
- Z. Zhelev, R. Jose, T. Nagase, H. Ohba, R. Bakalova, M. Ishikawa and Y. Baba, J. Photochem. Photobiol., B, 2004, 75, 99–105 CrossRef CAS PubMed.
- F. Iwata, E. M. Kuehl, G. F. Reed, L. M. McCain, W. A. Gahl and M. I. Kaiser-Kupfer, Mol. Genet. Metab., 1998, 64, 237–242 CrossRef CAS PubMed.
- L. W. Zhang and N. A. Monteiro-Riviere, Toxicol. Sci., 2009, 110, 138–155 CrossRef CAS PubMed.
- Y. Choi, K. Kim, S. Hong, H. Kim, Y. J. Kwon and R. Song, Bioconjugate Chem., 2011, 22, 1576–1586 CrossRef CAS PubMed.
- S. J. Tan, N. R. Jana, S. J. Gao, P. K. Patra and J. Y. Ying, Chem. Mater., 2010, 22, 2239–2247 CrossRef CAS.
- E. Frohlich, Int. J. Nanomed., 2012, 7, 5577–5591 CrossRef PubMed.
- W. Jiang, B. Y. S. Kim, J. T. Rutka and W. C. W. Chan, Nat. Nanotechnol., 2008, 3, 145–150 CrossRef CAS PubMed.
- J. Park, J. Nam, N. Won, H. Jin, S. Jung, S. Jung, S. H. Cho and S. Kim, Adv. Funct. Mater., 2011, 21, 1558–1566 CrossRef CAS.
- B. B. Zhang, X. H. Wang, F. J. Liu, Y. S. Cheng and D. L. Shi, Langmuir, 2012, 28, 16605–16613 CrossRef CAS PubMed.
- V. V. Breus, C. D. Heyes, K. Tron and G. U. Nienhaus, ACS Nano, 2009, 3, 2573–2580 CrossRef CAS PubMed.
- B. A. Kairdolf, M. C. Mancini, A. M. Smith and S. M. Nie, Anal. Chem., 2008, 80, 3029–3034 CrossRef CAS PubMed.
- M. A. Dobrovolskaia, P. Aggarwal, J. B. Hall and S. E. McNeil, Mol. Pharmaceutics, 2008, 5, 487–495 CrossRef CAS PubMed.
- V. Stone, H. Johnston and M. J. D. Clift, IEEE Trans. NanoBiosci., 2007, 6, 331–340 CrossRef.
- M. J. D. Clift and V. Stone, Theranostics, 2012, 2, 668–680 CrossRef CAS PubMed.
- E. L. Bentzen, I. D. Tomlinson, J. Mason, P. Gresch, M. R. Warnement, D. Wright, E. Sanders-Bush, R. Blakely and S. J. Rosenthal, Bioconjugate Chem., 2005, 16, 1488–1494 CrossRef CAS PubMed.
- C. D. Walkey, J. B. Olsen, H. B. Guo, A. Emili and W. C. W. Chan, J. Am. Chem. Soc., 2012, 134, 2139–2147 CrossRef CAS PubMed.
- M. J. D. Clift, B. Rothen-Rutishauser, D. M. Brown, R. Duffin, K. Donaldson, L. Proudfoot, K. Guy and V. Stone, Toxicol. Appl. Pharmacol., 2008, 232, 418–427 CrossRef CAS PubMed.
- N. Sakai, Y. Matsui, A. Nakayama, A. Tsuda and M. Yoneda, J. Phys.: Conf. Ser., 2011, 304, 012049 CrossRef.
- A. M. Derfus, W. C. W. Chan and S. N. Bhatia, Nano Lett., 2004, 4, 11–18 CrossRef CAS.
- C. Kirchner, T. Liedl, S. Kudera, T. Pellegrino, A. M. Javier, H. E. Gaub, S. Stolzle, N. Fertig and W. J. Parak, Nano Lett., 2005, 5, 331–338 CrossRef CAS PubMed.
- J. Y. Fan and P. K. Chu, Small, 2010, 6, 2080–2098 CrossRef CAS PubMed.
- H. Zheng, L. J. Mortensen and L. A. DeLouise, J. Biomed. Nanotechnol., 2013, 9, 382–392 CrossRef CAS PubMed.
- Y. Shen, M. Y. Gee, R. Tan, P. J. Pellechia and A. B. Greytak, Chem. Mater., 2013, 25, 2838–2848 CrossRef CAS.
- C. Park, D. H. Kim, M. J. Kim and T. H. Yoon, Bull. Korean Chem. Soc., 2008, 29, 303–304 CrossRef CAS.
- A. Shiohara, A. Hoshino, K. Hanaki, K. Suzuki and K. Yamamoto, Microbiol. Immunol., 2004, 48, 669–675 CrossRef CAS.
- W. W. Yu, E. Chang, R. Drezek and V. L. Colvin, Biochem. Biophys. Res. Commun., 2006, 348, 781–786 CrossRef CAS PubMed.
- J. K. Jiang, G. Oberdorster and P. Biswas, J. Nanopart. Res., 2009, 11, 77–89 CrossRef CAS.
- B. Pelaz, G. Charron, C. Pfeiffer, Y. L. Zhao, J. M. de la Fuente, X. J. Liang, W. J. Parak and P. del Pino, Small, 2013, 9, 1573–1584 CrossRef CAS PubMed.
- A. L. Jackson, S. R. Bartz, J. Schelter, S. V. Kobayashi, J. Burchard, M. Mao, B. Li, G. Cavet and P. S. Linsley, Nat. Biotechnol., 2003, 21, 635–637 CrossRef CAS PubMed.
- T. R. Brummelkamp, R. Bernards and R. Agami, Science, 2002, 296, 550–553 CrossRef CAS PubMed.
- L. F. Qi, W. J. Shao and D. L. Shi, J. Mater. Chem. B, 2013, 1, 654–660 RSC.
- J. M. Li, M. X. Zhao, H. Su, Y. Y. Wang, C. P. Tan, L. N. Ji and Z. W. Mao, Biomaterials, 2011, 32, 7978–7987 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra00288a |
| This journal is © The Royal Society of Chemistry 2014 |





