A facile one-step approach for the synthesis of uniform spherical Cu/Cu2O nano- and microparticles with high catalytic activity in the Buchwald–Hartwig amination reaction†
Abstract
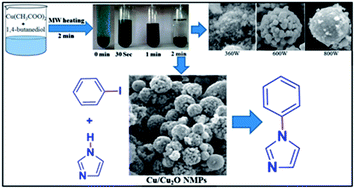
In the present work, we have developed a rapid, one step, calcination-free protocol for the synthesis of uniform spherical Cu/Cu2O nano/microparticles (NMPs). The synthesis of Cu/Cu2O NMPs was achieved by microwave irradiation of copper acetate as a precursor and 1,4-butanediol as a solvent in a few minutes. The prepared Cu/Cu2O NMPs gave 100% yield of uniform spherical morphology. 1,4-Butanediol plays a crucial role in reactions such as a solvent, reactant, stabilizer and capping agent which control the crystal morphology. The resultant product was characterized with the help of different techniques such as XRD, FEG-SEM, EDS, TEM, FT-IR, TPR, DSC-TGA, XPS and BET surface area analysis. The results confirm that the Cu/Cu2O NMPs had good crystallinity and were essentially pure. This is a simple, faster, inexpensive and additive-free protocol for synthesis of nanocrystalline Cu/Cu2O compared to the conventional method. These Cu/Cu2O NMPs showed excellent catalytic activity in the Buchwald–Hartwig amination coupling reaction. Notably the reaction does not require any ligand source, and requires low catalyst loading, and low temperature with catalyst recyclability.

 Please wait while we load your content...
Please wait while we load your content...