Nanoparticle tracking analysis of gold nanomaterials stabilized by various capping agents†
Abstract
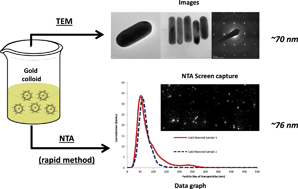
Gold colloidal nanomaterials have been synthesized using different methodologies and characterized by a novel nanoparticle tracking analysis (NTA) technique as compared to conventional Transmission Electron Microscopy (TEM) characterisation. Results prove that NTA is a highly useful, simple, efficient and rapid characterisation tool for Au nanoparticles and nanorods, providing a highly reliable and fast alternative to traditional characterisation techniques. This approach provides a very simple way of studying nanoparticles and some of their properties. Studies on the stabilizing/capping effect of a variety of biomass-derived compounds also show different possibilities for the use of polysaccharide to stabilise colloidal Au solutions.

 Please wait while we load your content...
Please wait while we load your content...