Biomass-based multifunctional fertilizer system featuring controlled-release nutrient, water-retention and amelioration of soil
Xinggang Wangac,
Shaoyu Lüa,
Chunmei Gaoa,
Xiubin Xua,
Yi Weia,
Xiao Baia,
Chen Fenga,
Nannan Gaoa,
Mingzhu Liu*a and
Lan Wub
aState Key Laboratory of Applied Organic Chemistry, Key Laboratory of Nonferrous Metal Chemistry and Resources Utilization of Gansu Province and Department of Chemistry, Lanzhou University, Lanzhou 730000, People's Republic of China. E-mail: mzliu@lzu.edu.cn; Fax: +86-931-8912582; Tel: +86-931-8912387
bCollege of Chemical Engineering, Northwest University for Minorities, Lanzhou 730030, People's Republic of China
cResearch Institute of Lanzhou Petrochemical Corporation, Petrochina Lanzhou Petrochemical Company, Lanzhou 730060, People's Republic of China
First published on 8th April 2014
Abstract
In an effort to enhance the efficiency of fertilizer use and minimize adverse environmental effects, a novel biomass-based, multifunctional controlled-release fertilizer (BMCF) was prepared to improve nutrient use efficiency and enhance crop production systems for more sustainable agriculture practices. The fertilizer design included natural attapulgite as a matrix, co-granulated ammonium zinc phosphate and urea as fertilizer core, cellulose acetate butyrate (CAB) as an inner coating, and a carboxymethyl chitosan-g-poly(acrylic acid)/attapulgite (CMCS-g-PAA/APT) superabsorbent composite as an outer coating. The effect of an APT matrix, CAB inner coating and superabsorbent composite outer coating on nutrient release rate was investigated. The influence of the BMCF on water-holding and water-retention capacity of soil samples was determined. The degradation behavior of the CMCS-g-PAA/APT outer coating in soil solution was evaluated. The experimental results indicated that the product prepared by a simple and economical method can effectively reduce N leaching loss and runoff, improve soil moisture retention capacity, and ameliorate soil acidity and alkalinity.
Introduction
Fertilizer has been used for many years to supply nutrients required in the greatest quantity by plants, and its availability, or lack thereof, limits growth and development of plants, crop yield, and primary production on a planetary scale.1 However, most of the fertilizer applied to the fields is lost by leaching into the groundwater or is evaporated into the atmosphere and cannot be used by crops, which may lead to serious environmental problems.2,3 To ensure the proper use of fertilizer by plants and overcome the negative effect on the environment, researchers have tried to find effective technique methods to achieve the novel defined goal of fertilizer use, that is, improving fertilizer use efficiency, reducing the frequency of their application, and minimizing the negative effects associated with over-dosage.4,5An effective way to overcome these shortcomings involves the use of slow- or controlled-release fertilizers (SRFs or CRFs), which is a valid technique to enhance fertilizer use efficiency and by thus alleviating environmental pollution caused by excessive fertilization while maintaining high crop yields of good quality.6 A great deal of effort has been devoted to the development of CRFs by investigators around the word. Actually, many CRFs such as urea-formaldehyde (UF) or sulphur-coated urea (SCU) were commercialized. However, UF suffered from high production cost caused by chemical reaction, while SCU required supplementary application of a sealant compound over the sulphur coating to seal minute fissures and pinholes in the coating. Moreover, acidification of soil owing to the use of sulphur can lead to serious problems afterward.7 Polymer-coated fertilizers (PCFs), physically prepared by encapsulating fertilizer core with various organic materials, are the major categories of the SRFs or CRFs.8,9 Petroleum-based, synthetic materials, such as polysulfone, polyolefine, polyvinyl chloride, polyurethane, polystyrene, glycidol ether and dicyclopentadiene have been reported to be used as potential coating materials for PCFs.10–12 However, the application of PCFs coating materials encountered a problem, because the remaining coating materials were non-biodegradable and non-renewable materials, and they can accumulate in soil, degrade soil fertility and become a new kind of environmental pollution. Therefore, the development of low-cost, renewable, biodegradable and environmentally friendly coating materials for PCFs will contribute to not only enhance fertilizer use efficiency, but also reduce adverse environmental impacts and agriculture production cost associated with PCFs application.13 Our research group has previously reported in regard to the production of slow- or controlled-release fertilizer from natural polysaccharide, such as starch,14 chitosan,15 and alginate16 based coating material. These materials are relatively cheaper, biodegradable and renewable, but their hydrophilicity and limited controlled release property are the weak points. Cellulose acetate butyrate (CAB) is a novel hydrophobic coating material with renewable and biodegradable properties which has been widely applied in biomedical, pharmaceutical, and agricultural fields.17 Due to its excellent properties, CAB will be a promising coating material of PCFs for developing an ideal controlled-release formulation.
The combination of irrigation and fertilizer applications together are another technology to increase crop yield.18 Many soils within arid regions have a limited water-retention capacity, and rain water may not effectively utilized by crops, which can affect nutrient uptake and hinder crop production.19 Superabsorbent composites are crosslinked three-dimensional networks of hydrophilic polymers that can capable of imbibing and retaining a large amount of water, with the absorbed water hardly removable even under some pressure.20 The possibility of their application in agricultural fields has been studied to alleviate agriculture problems, such as reducing irrigation water consumption, improving nutrient retention and ameliorating soil physical properties.21,22 However, its practical application in this field has been hindered because most of these superabsorbent polymers are petroleum-based monomers and cross-linkers, which are too expensive and non-biodegradable in soil.23 Among the numerous materials used for the preparation of superabsorbent polymers, natural polysaccharide, inorganic clay and biodegradable cross-linker have many advantages as compared with synthetic polymers, due to their nontoxic, biocompatible, economical, and biodegradable properties.24
Zinc (Zn) is one of the essential micronutrients required for normal plant growth and development, and Zn deficiency in crops can affect the metabolism of protein and decrease plants' resistibility in adverse environments.25 Ammonium zinc phosphate (NH4ZnPO4) is a slightly soluble Zn sources, can be used as a controlled-release Zn fertilizer to overcome concerns due to Zn toxicity and leaching loss. To the best we known, there is no literature for the development of NH4ZnPO4 as a controlled-release Zn fertilizer. Due to low contents of Zn are required by plants, the co-granulation of NH4ZnPO4 at low contents with macronutrients in nitrogen (N), phosphate (P), and potassium (K) products is preferred. Evidence shows that the absorption of Zn by plants can be enhanced up to 4-fold by increasing the nitrogen supply.26 Therefore, the co-granulation of NH4ZnPO4 with urea would have a positive synergistic effect on plant growth.
The objective of this study was to develop a multifunctional double-layer coated fertilizer on the basis of attapulgite (APT) clay as matrix, CAB film as inner-layer coating and carboxymethyl chitosan-g-poly(acrylic acid)/attapulgite (CMCS-g-PAA/APT) superabsorbent composite as outer layer coating, and evaluate its efficiency in controlling nutrient release, improving water-holding and water-retention capacity of soil, and regulating pH value of soil. The feasibility of NH4ZnPO4 as a source of micronutrient zinc fertilizer for incorporating in macronutrient formulations was studied. The physical and chemical properties of the coated fertilizer and its nutrient release characteristics were investigated.
Experimental section
Materials and methods
Chitosan (CS, with a degree of deacetylation of 0.9, Haidebei Co. Ltd., Jinan, China) with an average molecular weight of 5.0 × 105 and 2.0 × 104 were used to synthesis of carboxymethyl chitosan and N-maleyl chitosan (N-MCS), respectively. Acrylic acid (AA, analytic reagent, Tianjin Fine Chemical Reagent Factory, Tianjin, China) was used as a monomer. Natural attapulgite (APT, supplied by Gansu Haozhou APT Co., Ltd., Gansu, China) was milled and sieved through a 200-mesh screen before use. Cellulose acetate butyrate (CAB, Mn ∼ 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 16–19 mol% acetate, 35–39 mol% butyrate) and tributyl citrate (TBC, 98%) were obtained from Aladdin Reagent Co., Ltd. All other chemicals were analytical reagent and used as received.
000, 16–19 mol% acetate, 35–39 mol% butyrate) and tributyl citrate (TBC, 98%) were obtained from Aladdin Reagent Co., Ltd. All other chemicals were analytical reagent and used as received.
Synthesis of CMCS
Carboxymethyl chitosan (CMCS) was synthesized as the method reported in the literature.27 The substitution degree of CMCS was estimated by potentionmetric titration method28 and determined to be 0.95.Synthesis of N-MCS cross-linker
N-MCS was synthesized according to a method previously reported in the literature.29 Chitosan (CS, 0.5 g, and Mn = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) was dissolved in 40 mL of 2 wt% acetic acid aqueous solution with stirring and transferred to a three necked flask. Maleic anhydride (0.25 g) in a small amount of acetone was added into the solution, and then the reaction was carried out at room temperature for 8 h. The mixture was then poured into acetone to precipitate the polymer. The precipitate was washed with acetone and then freeze-dried. The substitution degree of N-MCS determined by 1H-NMR was 14.5%.
000 g mol−1) was dissolved in 40 mL of 2 wt% acetic acid aqueous solution with stirring and transferred to a three necked flask. Maleic anhydride (0.25 g) in a small amount of acetone was added into the solution, and then the reaction was carried out at room temperature for 8 h. The mixture was then poured into acetone to precipitate the polymer. The precipitate was washed with acetone and then freeze-dried. The substitution degree of N-MCS determined by 1H-NMR was 14.5%.
Synthesis of ammonium zinc phosphate fertilizer
The ammonium zinc phosphate was synthesized according to the literature.30 In short, the synthesis was carried out by adding the zinc sulfate solution (0.3 M) to the diammonium phosphate solution (0.3 M) at a constant flow under vigorous stirring at room temperature. Subsequently, the pH of the solution was adjusted to 9.0 by adding ammonia (25–28 wt%). The suspension was left for 2 h aging, and then the precipitate was filtered and vacuum dried to a constant weight.Synthesis of CMCS-g-PAA/APT superabsorbent composite
A series of superabsorbent composites from CMCS, AA, and APT were synthesized according to the following procedure. A certain amount of CMCS was first dissolved in 50 mL of distilled water in a four-necked flask equipped with a stirrer, reflux condenser, thermometer and gas inlet tube. Then, 0.010 g APS, 2.8 g of AA (partially neutralized by 5 mL of 7 mol L−1 NaOH aqueous solution), 0.03 g N-MCS cross-linker and 1.0 g APT were sequentially added. After being purged with nitrogen for 30 min, the solution was slowly heated to 60 °C and maintained at this temperature for 3 h. Finally, the resultant product was washed, sheared, dried, milled, screened and stored for further use. Moreover, CMCS-g-PAA superabsorbent polymer was prepared according to the same procedure.Preparation of coated BMCF granules
The procedures for the preparation of physically coated BMCF granules are as follows. First, an amount of NH4ZnPO4 and APT were ground to powder and mixed well. Then, the mixture was feed into a rotating disc with urea granules (about 1.0–1.3 mm in diameter) in batches. The fertilizer cores were obtained under water atomization. Subsequently, CAB and TBC were mixed at the ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) in ethyl acetate. Then, the solution (5%, w/v) was sprayed on the fertilizer cores to form the inner coating. The treatment is repeated several times until the desired thickness is reached. Finally, CMCS-g-PAA/APT powder (below 110 mesh) as the outer coating was coated on the surface of the granules under rotation. The coated granule products were dried in a dryer and screened to obtain the final products.
1 (w/w) in ethyl acetate. Then, the solution (5%, w/v) was sprayed on the fertilizer cores to form the inner coating. The treatment is repeated several times until the desired thickness is reached. Finally, CMCS-g-PAA/APT powder (below 110 mesh) as the outer coating was coated on the surface of the granules under rotation. The coated granule products were dried in a dryer and screened to obtain the final products.
Instrumental analysis
Fourier transforms infrared (FTIR) spectrum of APT, CMCS, CMCS-g-PAA and CMCS-g-PAA/APT was performed using a Nicolet NEXUS 670 FTIR Spectrometer (USA) with a KBr pellet in the range of 500–4000 cm−1. Scanning electron microscopy (SEM, JSM-5600LV, Japan) was used to observe the surface morphology of the samples. Before SEM observation, the surface of samples was coated with gold. The contact angle (CA) of the CAB coating for water was measured by the sessile drop method using a DSA-100 optical contact-angle meter (Kruss Co., Ltd., Germany). The CA value was determined automatically using the Laplace–Young fitting algorithm, and average CA values were obtained by measuring the sample at five different positions. The average diameter of double-layer coated fertilizer was measured by using a micrometer for 20 granules. The average crushing strength of BMCF was measured by compressing equipment for a certain amount of granules (2.8 ± 0.2 mm in diameter). The content of nitrogen in original and residual fertilizer was determined by an elemental analysis instrument (Germany Elemental Vario EL Corp., Model 1106) and the content of zinc and phosphorus was determined by an IRIS Advantage ER/S inductively coupled plasma emission spectrometer (TJA, USA).Controlled-release behavior of BMCF in soil
To study the controlled-release behavior of BMCF in soil, the following experiments were carried out: 1 g of BMCF was sealed into a non-woven plastic mesh bag and buried 6 cm beneath the surface of soil and kept in a beaker contained with 200 g of dry soil (below 26-mesh) at room temperature. The controlled experiment of untreated urea (the nitrogen content of urea was the same as that of 1 g BMCF) was also carried out. Throughout the experiment, water-holding ratio of the soil was maintained at 30% by weighing and adding tap water if necessary, periodically. After 1, 3, 5, 10, 15, 20, 25, and 30 days, the mesh bags were retrieved and placed in a glass dish and then dried at room temperature overnight to estimate the contents of N, P and Zn. For eight measurements, eight beakers were prepared at the same time. The remaining nutrient content of N in residual fertilizer was determined by an elemental analysis, the content of P and Zn was determined by an Inductively-Coupled Plasma Atomic-Emission Spectrometry. The soil used in this study is a representative sample of Lanzhou, which lies in the northwest of China and is a semiarid region.Measurement of water-holding and water-retention capacity of soil with BMCF
The soil samples used for the experiment were sandy loam soils collected from the field at the Lanzhou. The experiment was conducted at a relative humidity ranged from 41% to 49% at 20 ± 3 °C, and the average daily evaporation of 5.4 mm. The water-holding capacity was measured for four different treatments: a control, 200 g of dry soil only (A), 200 g of dry soil mixed with 1.0 g of BMCF (B), 200 g of dry soil mixed with 2 g of BMCF (C), and 200 g of dry soil mixed with 3 g of BMCF (D). Each sample was placed into a poly(vinyl chloride) tube of 4.5 cm in diameter. The bottom of the tube was sealed with two layers of nylon fabric (200-mesh) and weighed (marked W0). The soil samples were slowly drenched by tap water from the top of the tube until water seeped out from the bottom. After that there was no seeping water at the tube bottom, the tube was weighed again (marked W1). The water-holding capacity (WH%) of the soil was calculated from eqn (1):
 | (1) |
The water-retention capacity of BMCF was measured for three different treatments: a control, 200 g of dry soil only (A), 200 g of dry soil mixed with 1 g of BMCF (B), and 200 g of dry soil mixed with 2 g of BMCF (C). Soil samples were placed into a poly(vinyl chloride) tube of 4.5 cm in diameter. The bottom of the tube was sealed with two layers of nylon fabric (200-mesh) and weighed (marked W0). The soil samples were slowly drenched by tap water from the top of the tube until water seeped out from the bottom. After that there was no seeping water at the tube bottom, the tube was weighed again (marked W1). The columns were kept under identical conditions at room temperature, and weight every 3 days (Wi), the measurement was obtained after a period of 30 days. The water-retention ratio (WR%) of the soil was calculated from eqn (2):
 | (2) |
The effect of CMCS-g-PAA/APT superabsorbent composite on the pH value of soil
Simulated soil solution samples with different pH values were adjusted with 0.1 M HCl or NaOH aqueous solution. 0.1 g of CMCS-g-PAA/APT sample was immersed in 100 mL of soil solution with different pH values for 90 min. After the swollen superabsorbent composite was filtered, the pH value of the filtrate was measured with a pH meter (pHS-3B, Shanghai Precision Scientific Instrument Co., Ltd., Shanghai, China).Degradation of CMCS-g-PAA/APT outer coating materials
The degradation behavior of CMCS-g-PAA/APT superabsorbent composite with different contents of cross-linker was studied. In order to simulate natural conditions, the degradation of superabsorbent composite was monitored by weight loss in soil solution. The soil solution was generally prepared by a centrifugation method.31 In a typical experiment, 100 g of soil was extracted with 500 mL of distilled water over 24 h at room temperature, and the pH of the extracts was measured. Then, the sample was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 3 min. The degradation of CMCS-g-PAA/APT superabsorbent composite in soil solution were performed by incubating pre-weighed dried slice (6.5–7.5 mm in diameter and 1–1.5 mm in thickness) of samples in 50 mL of soil solution at the ambient temperature. After incubation for a predetermined time period, the samples were removed from the solution by filtration, washed repeatedly with distilled water, and dried at 40 °C to a constant weight. The extent of degradation was calculated by the following eqn (3):
000 rpm for 3 min. The degradation of CMCS-g-PAA/APT superabsorbent composite in soil solution were performed by incubating pre-weighed dried slice (6.5–7.5 mm in diameter and 1–1.5 mm in thickness) of samples in 50 mL of soil solution at the ambient temperature. After incubation for a predetermined time period, the samples were removed from the solution by filtration, washed repeatedly with distilled water, and dried at 40 °C to a constant weight. The extent of degradation was calculated by the following eqn (3):
 | (3) |
Statistical analysis
At least three determinations were made for all experiments, except where indicated otherwise. The average value was calculated for each treatment. Analysis of variance among treatments and mean separation test (Duncan's multiple range test, Pearson correlation, and least significant difference test [LSD]) were performed using Statistical Analysis System (SAS) package version 9.2 (2010, SAS Institute Inc.). The difference among means and correlation coefficients were considered significant when P < 0.05.Results and discussion
FTIR analysis
The FTIR spectra of APT (Fig. 1a), CMCS (Fig. 1b), CMCS-g-PAA (Fig. 1c), and CMCS-g-PAA/APT (Fig. 1d) were shown in Fig. 1. As shown in Fig. 1a, the absorption peaks observed at 3617 and 3552 cm−1 (hydroxyl stretching vibration), 1030 cm−1 (silicon–oxygen stretching vibration), 525 and 472 cm−1 (silicon–oxygen bending vibration) were characteristic absorption peaks of APT. According to Fig. 1b, the peaks observed at 3435 cm−1 (hydroxyl stretching vibration), 2921 cm−1 (methylene), 1608 cm−1 (carbonyl stretching vibration) and 1059 cm−1 (β-1,4-glycosidic bond) were characteristic absorption peaks of carboxymethyl chitosan. The peak at 1719 cm−1 in Fig. 1c and the peak at 1717 cm−1 in Fig. 1d were ascribed to carbonyl stretching vibration of acrylate group. Comparing Fig. 1c with b, it could be observed that the absorption peaks at 1418, 1326 and 716 cm−1 attributed to in-plane and out-plane bending vibration of –OH groups on carboxymethyl chitosan almost disappeared after reaction. These changes were also presented in Fig. 1d. It was suggested that the graft polymerization between hydroxyl groups on carboxymethyl chitosan and the acrylic acid monomers took place.32 As compared with Fig. 1a, the absorption peaks of APT at 3617, 3552 cm−1 disappeared, and the peaks at 1030, 525, and 472 cm−1 weakened in Fig. 1d. It was indicated that the graft copolymerization between Si–OH groups on the surface of APT and acrylic acid occurred during the reaction. From the above analysis of FTIR information, it can be concluded that the CMCS-g-PAA/APT has been successfully prepared. A proposed reaction mechanism for the synthesis of CMCS-g-PAA/APT superabsorbent composite is presented in Scheme 1. Initially, the initiator APS is decomposed under heating to generate sulfate anion radicals. Thereafter, the resulting anion radical abstracts hydrogen atom from one of the functional groups (–OH or –NH2) on CMCS backbone to form macroradicals. Finally, these macroradicals initiate the grafting of vinyl monomer AA onto CMCS backbone. During the polymerization process, the nanorods of APT would be dispersed in the polymer matrix, and the crosslinker N-MCS react synchronously with polymer chains during the chain propagation to form a superabsorbent composite with three-dimensional polymeric networks. | ||
| Scheme 1 Proposed reaction mechanism pathway for the synthesis of the CMCS-g-PAA/APT superabsorbent composite. | ||
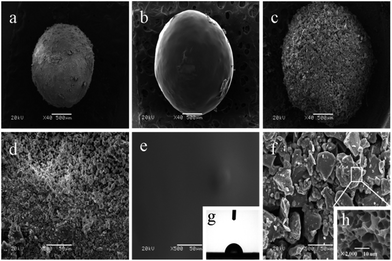
Morphology of the coated fertilizer granules
Fig. 2 shows the morphology of the fertilizer core (Fig. 2a), the fertilizer core with the inner coating (Fig. 2b), and the final product with the inner and outer coating (Fig. 2c). The following are enlarged figures of their surface morphology. The illustrations are the profile of water on the surface of the CAB coating film and the freeze-dried swollen CMCS-g-PAA/APT superabsorbent composite. Meanwhile, the contact angle of CAB film is measured to be 83° for water. As can be seen from Fig. 2b, the surface of the inner coating fertilizer is smooth due to CAB film, which could hinder the moisture from penetrating through it into the fertilizer core. Fig. 2c shows small particles of CMCS-g-PAA/APT superabsorbent composite covered the surface of the fertilizer, which is coarse and porous. So, water can be absorbed quickly by the product because it has a large specific surface area. The physical and chemical properties of the double-layer coated fertilizer are presented in Table 1.| Characteristics | Value |
|---|---|
| Nitrogen content | 26.9% (urea) + 0.2% (NH4+) |
| Zinc content | 0.72% |
| Phosphorus (P2O5) content | 1.77% |
| APT content | 35.5% |
| CAB content | 5.3% |
| CMCS-g-PAA/APT content | 8% |
| Average crushing strength | 17.9 N |
| Diameter of granules | 2.5–3 mm |
Controlled-release behavior of BMCF in soil
One of the most important characteristics of the coated CRFs is the controlled-release property. As shown in Fig. 3a, the release rate of untreated urea is very quickly. More than 98.5% of its constitutive nitrogen was released within 24 h. As compared with the untreated urea, the release rate of nitrogen from CAB film single layer coated fertilizer shown in Fig. 3b decreased sharply, and about 15.6, 85.6, and 96.8% of nitrogen released from the coated fertilizer by the 1, 15, and 30 days, respectively. In addition, the effect of multiple coatings of the fertilizer granules on the release of nutrients was studied. Fig. 3b and c illustrate the influence of the number of sprayed CAB layers on the release rate of nutrients. It can be observed that the nitrogen release rate from CAB film single layer coated granules was about 38.3, 85.3, and 96.5%, whereas the triple coated granules only 15.4, 70.3, and 87.5% of N released for 3, 15, and 30 days, respectively. The reduction of the multicoated granules' release rate was caused by a more compact structure of the coating in comparison with that of the single-layer coating. Moreover, the study results indicated that the release rate of nutrient from coated granules decreased with increasing thickness of applied CAB coating film, but was still higher than that of CAB film and CMCS-g-PAA/APT superabsorbent composite double-layer coated fertilizer granules (BMCF). As shown in Fig. 3d, about 9.2, 53.1, and 81.4% of nitrogen released from the BMCF after 3, 15, and 30 days, respectively. Ni et al.16 previously reported a multifunctional slow-release organic–inorganic compound fertilizer (MSOF) with an inner coating of Ca2+ crosslinked alginate, and the outer coating of sodium alginate-g-poly(acrylic acid-co-acrylamide)/humic acid superabsorbent polymer. The soil incubation experiments revealed that the nitrogen almost released completely within 30 days. In comparison, the release rate of nitrogen from BMCF was found to be slower. As shown in Fig. 3e, the nutrient Zn from the BMCF has a lower release rate than N, only 41.2% released within 30 days. This can be attributed to the low water solubility of NH4ZnPO4 in the fertilizer core and the electrostatic interaction between Zn2+ and negatively charged –COO− on the CMCS-g-PAA/APT polymeric networks. The above results indicated that the BMCF system can effectively controlled the release rate of nutrients.The release mechanism of BMCF in soil could be illustrated as follows. The outer coating material of BMCF was CMCS-g-PAA/APT superabsorbent composite, which could be slowly swollen by soil solution and gradually transformed into hydrogel after the coated fertilizer was added into soil. The free water in the superabsorbent composite outer coating layer subsequently transferred to the inner coating layer of the CAB capsule. Water vapour soaks and penetrates through the inner coating layer of CAB capsule, dissolves a small fraction of nutrients. The dissolved nutrients diffuse out from the CAB inner coating layer, enter into the CMCS-g-PAA/APT outer coating layer, which occurs at a rate proportional to the nutrient concentration gradient.33 During this final stage, the CAB inner coating layer acts as an impermeable physical barrier, restraining the release of nutrients. It was observed that the increase of the number of sprayed CAB coating layers caused a decrease in the release rate of nitrogen. This is because when the thickness of CAB film was increased, the diffusion of water through the film is slowed down and the diffusion pathway for urea molecules is increased with the increase of the film coating thickness. Therefore, the release rate of nutrients can be controlled by adjusting the thickness of the CAB inner coating. When nutrients diffused from the CAB inner coating to the outer coating layer, they may be easily adsorbed by the charged CMCS-g-PAA/APT superabsorbent composite and released into the soil through dynamic water exchange between the BMCF and the soil.34 It is obvious that the outer coating of CMCS-g-PAA/APT superabsorbent composite played an important role in retarding nutrient release. When the fertilizer cores were coated with CAB (50–100 μm) and CMCS-g-PAA/APT (400–500 μm) double-layer coatings, the combine of hydrophobic effect of CAB inner coating and retarding effect of CMCS-g-PAA/APT outer coating played together to control the nutrient release, which led to a better controlled release property than that of CAB coated or untreated fertilizer. The nutrient Zn in NH4ZnPO4 of BMCF has an excellent controlled-release property, which is available in the soil as to be assimilated by plants for a long period of time. This is attributed to the fact that the NH4ZnPO4 is a slightly soluble compound fertilizer and the retarding effect of double layer coating. Therefore, NH4ZnPO4 could be used as Zn sources for co-granulation with macronutrient fertilizers such as urea to produce controlled-release compound fertilizer.
In order to investigate the nutrients release mechanism from coated BMCF granules in soil, the experimental data was fitted to the Ritger–Peppas35 (4) and Al-Zahrani36 (5) equation as follows.
 | (4) |
 | (5) |
| Nutrients | n | K | R2 | D (mm2 per day) |
|---|---|---|---|---|
| N | 0.53 | 7.63 | 0.9905 | 0.021 |
| Zn | 0.57 | 5.69 | 0.9977 | 0.012 |
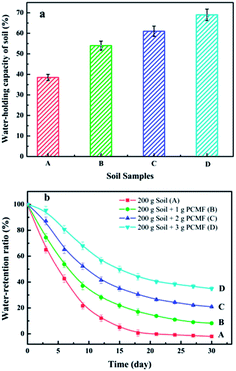
Water-holding and water-retention capacity of soil with BMCF
Sandy soils from arid and semiarid regions having low water-holding capacity which results in excessive drainage of rain and irrigation water below the root zone, leading to poor water use efficiency by crops.38 Therefore, superabsorbent composites have been developed and used in agriculture to enhance soil water-holding capacity.21 In this study, the application of BMCF to improve the water-holding capacity of soil was investigated. For the soil samples, (A) 200 g soil only (control), (B) 200 g of soil mixed with 1 g of BMCF, (C) 200 g of soil mixed with 2 g of BMCF, and (D) 200 g of soil mixed with 3 g of BMCF, the largest WHs were 38.5 ± 1.5, 54 ± 2.2, 61 ± 2.5, and 69 ± 2.8%, respectively, as shown in Fig. 4a. Compared with the control (soil without BMCF), the addition of BMCF can effectively improve the water-holding capacity of soil. Moreover, the water-holding capacity of the soil increased with dosage of BMCF. In this regard, the application of BMCF could effectively enhance the water-holding capacity of soil.Furthermore, the water-retention capacity of soil with different addition amount of BMCF was also studied. As shown in Fig. 4b, we can observed that the soil samples addition of it is greater than that of the control group (soil without BMCF), and the water-retention capacity of the soil goes up with increasing the dosage of BMCF. After 15 days test, the water-retention capacity of the soil samples are found to be 5.1, 21.8, 35.0 and 49.8% of the soil samples A, B, C and D, respectively. After 18 days, the soil sample without BMCF had almost given off all water, but the soil samples B, C and D could still retained 17.1, 30.4 and 44.1% of soil moisture, respectively. Clearly, compared with the control (soil without BMCF), the soil with the addition of BMCF could effectively improve the water-retention capacity of soil. The reason is that the outer coating of CMCS-g-PAA/APT superabsorbent composite could absorb and retain large amounts of water and allow to gradually releasing it out during the soil lack of moisture. The soil by the addition of BMCF which would be like a subminiature reservoir to retain and supply moisture for plants over a considerable period of time as the soil underwent dry periods. Therefore, the application of BMCF could be an important agricultural practice to enhance water-retention capacity of soils in arid areas by providing drought protection and reducing irrigation water consumption.
Effect of CMCS-g-PAA/APT superabsorbent composite on soil acidity and alkalinity
It was reported that soil nutrients are most available to plant roots when soil pH is in the range of 5.5–7.0, and soil microbial biomass and microbial activity tend to stabilize at this pH range.39 In this study, the effect of CMCS-g-PAA/APT superabsorbent composite on pH value of soil is investigated, as shown in Fig. 5.As can be seen from Fig. 5, all the pH values of the simulated soil solution are adjusted to about 7.0 after treating with the superabsorbent composite. The result indicates that the CMCS-g-PAA/APT superabsorbent composite can buffer soil acidity or alkalinity so as to develop an optimal pH environment for plant growth. This is because of CMCS-g-PAA/APT superabsorbent composite with large amounts of –COOH and –COO− groups. Under alkaline conditions, the –COOH groups can react with OH− of the soil solution, whereas, in acidic conditions, the –COO− groups can react with H+ of the soil solution.40 Therefore, the CMCS-g-PAA/APT superabsorbent composite prepared in this study can not only absorb water to enhance plants survival in arid conditions, but also adjust pH value of soil as a kind of soil amendment.
Degradation behavior of CMCS-g-PAA/APT outer coating
The degradation property of the coating material is of great importance in designing the coated CRFs. However, the cross-linker is a critical factor in the degradation of polymeric matrix.41 In this study, the degradation rate of superabsorbent composite with different biodegradable cross-linker (N-MCS) contents was investigated by monitoring the weight loss in soil solution. The results obtained were depicted in Fig. 6. It is observed that the extent of degradation of CMCS-g-PAA/APT superabsorbent composite was decreased with increasing cross-linker content, which was consistent with the swelling degree (SD) of the superabsorbent composite (SD was 305, 265, and 200 g g−1, respectively). When the slice of CMCS-g-PAA/APT was immersed in the soil solution, it swelled, and the interchain spacing expanded and formed a three-dimensional polymeric structure, which facilitated the interaction of superabsorbent composite with soil microorganisms. Thus, the larger the swelling degree of superabsorbent composite, the more adequately it contacts with soil microorganism and the faster the degradation rate. Primarily, CMCS and N-MCS can be biodegraded into small molecular fragments by microorganisms and released into the soil solution.42 Meanwhile, with the degradation occurred, and the cross linkages between N-MCS and two PAA chains broke homogeneously throughout the degradation process. This ongoing break of cross-links within the polymer decreased the cross-linking density of the polymeric network and eventually led to the disintegration of the composite hydrogel. Therefore, the higher content of N-MCS cross-linker could result in the lower extent of degradation of CMCS-g-PAA/APT superabsorbent composite. As shown in the Fig. 6, increased weight loss with time indicated the biodegradability of the superabsorbent composite. After degradation for 50 days, the final percentage of degradation of CMCS-g-PAA/APT superabsorbent composites reached to 52.7, 38.8, and 31.5% for N-MCS content of 1, 2, and 3%, respectively. The introduction of CMCS, N-MCS and APT into the superabsorbent composite effectively imparted an ease of degradation to coating material, yielding degradation residues that serve as nutrient-rich fertilizers for plants. Therefore, the new approach prepared superabsorbent composite exhibit significantly improved coating material degradation properties, which can be applied in agriculture and horticulture as a new kind of coating materials to alleviate the environmental pollution.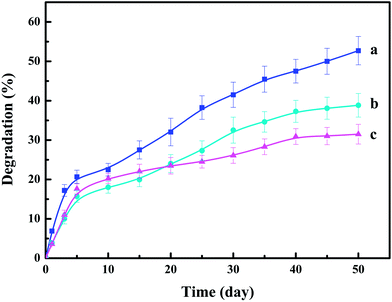 | ||
| Fig. 6 Degradation of CMCS-g-PAA/APT superabsorbent composite with different contents of cross-linker in soil solution: (a) 1% N-MCS; (b) 2% N-MCS; (c) 3% N-MCS. | ||
Conclusions
A novel multifunctional controlled-release fertilizer with double-layer coatings was prepared. The nutrients (H2NCONH2 and NH4ZnPO4) were entrapped in the APT clay matrix as fertilizer core. Cellulose acetate butyrate (CAB) and carboxymethyl chitosan-g-poly(acrylic acid)/attapulgite (CMCS-g-PAA/APT) superabsorbent composite were used as coatings. The composition analysis results showed that the product contained 27.1% N, and 0.72% Zn. The obtained product shows excellent performances in controlled-release properties: nutrient N released 69.1% within 25 days, micronutrient Zn released 41.3% within 30 days. Above all the introduction of APT can slow fertilizer release rate and reduce the production cost. The addition of BMCF could significantly enhance the water-holding and water-retention capacity of the soil. The untreated soil loses almost all of the water after 18 days, whereas 30.4% of the initial absorbed water of the soil mixed with 2% BMCF can still retained. Furthermore, the CMCS-g-PAA/APT outer coating could be used as a soil amendment to regulate the pH value of soil to a suitable level. In addition, the introduction of CMCS, APT and N-MCS into the as-prepared superabsorbent composite effectively imparted an ease of degradation to the outer coating material. Therefore, the as-prepared multifunctional controlled-release fertilizer exhibited promising application for sustainable development of modern agriculture and horticulture.Abbreviations used
| CMCS | Carboxymethyl chitosan |
| APT | Attapulgite |
| N-MCS | N-Maleyl chitosan |
| SD | Swelling degree |
| APS | Ammonium persulfate |
| PCFs | Polymer-coated fertilizers |
| CRFs | Controlled-release fertilizers |
| SRFs | Slow-release fertilizer |
| TBC | Tributyl citrate |
| AA | Acrylic acid |
| N | Nitrogen |
| P | Phosphorus |
| Zn | Zinc |
| WR | Water-retention |
| CAB | Cellulose acetate butyrate |
| WH | Water-holding |
Acknowledgements
The authors gratefully acknowledge the financial support of the National Natural Science Foundation of China (grant no. 51273086, 31260500), Special Doctorial Program Fund from the Ministry of Education of China (grant no. 20090211110004), and the Fundamental Research Funds for the Central Universities (grant no. lzujbky-2013-64).References
- R. A. Gutiérrez, Science, 2012, 336, 1673 CrossRef PubMed.
- D. Fowler, M. Coyle, U. Skiba, M. A. Sutton, J. N. Cape, S. Reis, J. Sheppard, A. Jenkins, B. Grizzetti, J. N. Galloway, P. Vitousek, A. Leach, A. F. Bouwman, K. Butterbach-Bahl, F. Dentener, D. Stevenson, M. Amann and M. Voss, Philos. Trans. R. Soc., B, 2013, 368, 20130164 CrossRef PubMed.
- Y. Y. Zhang, J. F. Liu, Y. J. Mu, S. W. Pei, X. X. Lun and F. H. Chai, Atmos. Environ., 2011, 45, 2956 CrossRef CAS PubMed.
- S. Chien, L. I. Prochnow and H. Cantarella, Adv. Agron., 2009, 102, 267 CAS.
- A. Shaviv, in Proc.-Int. Fert. Soc., International Fertilizer Society, York, 2001, vol. 469, pp. 1–23 Search PubMed.
- S. H. Chien, L. I. Prochnow and H. Cantarella, Adv. Agron., 2009, 102, 267 CAS.
- B. S. Ko, Y. S. Cho and H. K. Rhee, Ind. Eng. Chem. Res., 1996, 35, 250 CrossRef CAS.
- D. Davidson and F. X. Gu, J. Agric. Food Chem., 2012, 60, 870 CrossRef CAS PubMed.
- S. P. Jin, Y. G. Yue, L. Feng, Y. Q. Han, X. H. Yu and Z. H. Zhang, J. Agric. Food Chem., 2011, 59, 322 CrossRef CAS PubMed.
- M. S. Al-Zahrani, Ind. Eng. Chem. Res., 2000, 39, 367 CrossRef.
- M. Tomaszewska and A. Jarosiewicz, J. Agric. Food Chem., 2002, 50, 4634 CrossRef CAS PubMed.
- S. M. Tao, J. Liu, K. M. Jin, X. Y. Qiu, Y. Zhang, X. Q. Ren and S. W. Hu, J. Appl. Polym. Sci., 2011, 120, 2103 CrossRef CAS.
- Y. C. Yang, Z. H. Tong, Y. Q. Geng, Y. C. Li and M. Zhang, J. Agric. Food Chem., 2013, 61, 8166 CrossRef CAS PubMed.
- M. Y. Guo, M. L. Liu, F. L. Zhan and L. Wu, Ind. Eng. Chem. Res., 2005, 44, 4206 CrossRef CAS.
- L. Wu and M. Z. Liu, Carbohydr. Polym., 2008, 72, 240 CrossRef CAS PubMed.
- B. L. Ni, S. Y. Lü, L. H. Xie and Y. F. Wang, J. Agric. Food Chem., 2011, 59, 10169 CrossRef CAS PubMed.
- K. Edgar, C. Buchanan and J. Debenham, Prog. Polym. Sci., 2001, 26, 1605 CrossRef CAS.
- H. Y. Hu, T. Y. Ning, Z. J. Li, H. F. Han, Z. Z. Zhang, S. J. Qin and Y. H. Zheng, Field Crops Res., 2013, 142, 85 CrossRef PubMed.
- S. E. Jacobsen, C. R. Jensen and F. Liu, Field Crops Res., 2012, 128, 34 CrossRef PubMed.
- C. C. Liu, L. Yu, Y. T. Zhang, B. Zhang, J. D. Liu and H. Q. Zhang, RSC Adv., 2013, 3, 13756 RSC.
- M. Zohuriaan-Mehr, H. Omidian and S. Doroudiani, J. Mater. Sci., 2010, 45, 5711 CrossRef CAS.
- W. Bai, H. Zhang, B. Liu, Y. Wu and J. Song, Soil Use Manage., 2010, 26, 253 CrossRef.
- E. Karada, Ö. Üzüm and D. Saraydin, Eur. Polym. J., 2002, 38, 2133 CrossRef.
- B. L. Ni, S. Y. Lü and M. Z. Liu, Ind. Eng. Chem. Res., 2012, 51, 12993 CrossRef CAS.
- M. Broadle, P. White and J. Hammond, New Phytol., 2007, 173, 677 CrossRef PubMed.
- E. B. Erenoglu, U. B. Kutman, Y. Ceylan, B. Yildiz and I. Cakmak, New Phytol., 2011, 189, 438 CrossRef CAS PubMed.
- A. Zamani, D. Henriksson and J. M. Taherzadeh, Carbohydr. Polym., 2010, 80, 1091 CrossRef CAS PubMed.
- X. P. Kong, Carbohydr. Polym., 2013, 88, 336 CrossRef PubMed.
- Y. M. Dong, W. Mao, H. W. Wang, Y. Q. Zhao, X. J. Li, D. X. Bi, L. L. Yang, Q. Ge and X. M. Fang, Polym. Int., 2006, 55, 1444 CrossRef CAS.
- J. McCullough, E. City and M. Salut-sky, US Pat., 3141732, 1964.
- B. E. Davies and R. I. Davies, Nature, 1963, 198, 216 CrossRef.
- Y. Chen, Y. F. Liu, H. L. Tang and H. M. Tan, Carbohydr. Polym., 2010, 81, 365 CrossRef CAS PubMed.
- Y. C. Yang, M. Zhang, Y. C. Li, X. H. Fan and Y. Q. Geng, J. Agric. Food Chem., 2012, 60, 11229 CrossRef CAS PubMed.
- L. H. Xie, M. Z. Liu, B. L. Ni and Y. F. Wang, J. Agric. Food Chem., 2012, 60, 6921 CrossRef CAS PubMed.
- P. L. Ritger and N. A. A. Peppas, J. Controlled Release, 1987, 5, 37 CrossRef CAS.
- S. M. Al-Zahrani, Int. J. Eng. Sci., 1999, 37, 1299 CrossRef.
- P. Colombo, R. Bettini, P. L. Catellani, P. Santi and N. A. Peppas, Eur. J. Pharm. Sci., 1999, 9, 33 CrossRef CAS.
- J. Yu, J. G. Shi, P. F. Dang, A. I. Mamedov, I. Shainberg and G. J. Levy, Soil Sci. Soc. Am. J., 2011, 76, 1758 CrossRef.
- A. S. Wang, J. S. Angel, R. L. Chaney, T. A. Delorme and R. D. Reeves, Plant Soil, 2006, 281, 325 CrossRef CAS.
- W. B. Wang and A. Q. Wang, Carbohydr. Polym., 2010, 80, 1028 CrossRef CAS PubMed.
- C. Cha, R. H. Kohman and H. Kong, Adv. Funct. Mater., 2009, 19, 3056 CrossRef CAS.
- Y. N. Phang, S. Y. Chee, C. O. Lee and Y. L. The, Polym. Degrad. Stab., 2011, 96, 1653 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |