Celastrol-modified TiO2 nanoparticles: effects of celastrol on the particle size and visible-light photocatalytic activity†
Abstract
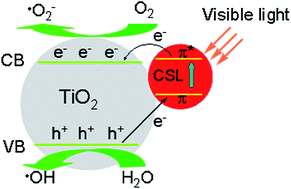
In this study, celastrol (CSL), a triterpene compound which is derived from the Chinese medicinal plant Tripterygium wilfordii, was used to synthesize CSL-modified TiO2 nanoparticles by a hydrothermal treatment method. The nanoparticles phase, morphology, surface structures, as well as the visible-light photocatalytic activities were studied. The modification of CSL does not alter the crystalline structure of the TiO2 but can prevent TiO2 from further aggregating and provides photosynthesis pigments as an in situ dye-sensitizing source. Compared to pure TiO2, the resulting CSL-modified TiO2 nanoparticles exhibit enhanced visible-light photocatalytic activity and showed an excellent cyclic stability in the decomposition of methylene blue (MB). In addition, the photocatalytic degradations of MB were all demonstrated to follow a first-order kinetic model. The enlarged specific surface area and CSL-modified surface improved the visible-light photocatalytic activity of nanoparticles.

 Please wait while we load your content...
Please wait while we load your content...