Hydrocarbon and hydrogen-rich syngas production by biomass catalytic pyrolysis and bio-oil upgrading over biochar catalysts
Shoujie Ren,
Hanwu Lei*,
Lu Wang,
Quan Bu,
Shulin Chen and
Joan Wu
Bioproducts, Sciences and Engineering Laboratory, Department of Biological Systems Engineering, Washington State University, Richland, WA 99354-1671, USA. E-mail: hlei@wsu.edu; Fax: +509-372-7690; Tel: +1 5093727628
First published on 3rd February 2014
Abstract
The focus of this study is to investigate the influences of biochar as a catalyst in biomass pyrolysis and bio-oil upgrading. The biochar catalyst enhanced the syngas and improved the bio-oil quality in biomass pyrolysis. The high concentrations of phenols (46 area%) and hydrocarbons (16 area%) were obtained from torrefied biomass catalytic pyrolysis over biochar catalysts. High-quality syngas enriched in H2, CO, and CH4 was observed. The amounts of H2 and CO in syngas were up to 20.43 vol% and 43.03 vol% in raw biomass catalytic pyrolysis, and 27.02 vol% and 38.34 vol% in torrefied biomass catalytic pyrolysis. Thermal gravimetric (TG) analysis showed that the raw and recycled biochar catalysts had good thermal stability. Upgraded bio-oil was dominated by phenols (37.23 area%) and hydrocarbons (42.56 area%) at high biochar catalyst loadings. The biochar catalyst might be used as a cost-competitive catalyst in biomass conversion and bio-oil upgrading.
1. Introduction
Biomass pyrolysis is a thermo-chemical process conducted at the temperature of 350–600 °C under inert conditions.1 The primary products of biomass fast pyrolysis are bio-oils that are carbon-based liquids with some properties similar to those of petroleum fuel, such as low solid content and low viscosity.2 Bio-oils also have low nitrogen and sulfuric contents.Still, biomass pyrolysis has certain disadvantages. For instance, the crude bio-oils from biomass pyrolysis contain high content of oxygen, up to 35–40 wt% and much higher than that of petroleum. The crude bio-oils are acidic and reactive due to the existence of organic acids. The high contents of oxygen and organic acids make the bio-oils unstable and immiscible with gasoline. Furthermore, the bio-oils contain few hydrocarbons that are dominant chemicals in petroleum, and, the heating value of bio-oils is about 17 MJ kg−1, which is lower than half of that of crude oil. Hence, the bio-oils need to be upgraded to transportation fuels by removing organic acids, reducing oxygen content, and improving hydrocarbon content.
A number of technologies for bio-oil upgrading have been developed and reported, including hydrotreating, hydrocracking, esterification, emulsification, and steam reforming.3–8 Among these technologies, hydrotreating and hydrocracking should be operated under pressure conditions and consume large amount of hydrogen.3,4 Esterification and emulsification need solvents that will increase the cost of process.5,6 Steam reforming is a complicated process with high reaction temperature.7,8 Recently, biomass catalytic pyrolysis and bio-oil catalytic cracking are drawing more interest as they do not consume hydrogen and can be conducted at atmospheric or low-pressure conditions. In previous studies, scientists have investigated biomass catalytic pyrolysis and catalytic cracking for bio-oil and vapors using different catalysts, such as zeolite-based catalysts,9 activated alumina,10 fluid catalytic cracking (FCC) catalysts,11,12 and transition metal catalysts (Fe/Cr).11 They reported that the oxygen content was significantly reduced and the selectivity for hydrocarbons was increased. However, the catalysts used in biomass catalytic pyrolysis and catalytic cracking are expensive as they need to be purchased or synthesized by using metals or expensive precious metals. Therefore, it is necessary to develop cost-competitive catalysts to improve the efficiency of biomass conversion.
Biochar is one of the main co-products in biomass pyrolysis and gasification. With the development of biomass thermo-chemical conversion, abundant biochar will be produced. The analysis of biochar properties indicated that biochar has relatively high porosity and surface area, and it contains high minerals such as K, Ca, and P and functional groups on the surface.13–15 These properties make the biochar interesting as adsorbent, catalyst support and catalyst. Application of biochar has been widely investigated in areas of soil amendment,16 water treatment,17 activated carbon production,18 and catalyst support.19 Recently, biochar-supported catalysts were developed for biodiesel production,19,20 catalytic esterification,21 biogas reforming,22 and biomass hydrolysis.23 However, studies using biochar as a catalyst in biomass pyrolysis and bio-oil upgrading have been lacking.
In our previous research, woody pellets biomass has been successfully pyrolyzed, producing comparable liquid yield by microwave heating.24 The torrefied woody pellet biomass through microwave pyrolysis showed the enhancement of sugar, phenolic chemicals, and hydrocarbons in bio-oil and improvement in syngas quality.25 This paper present the continued study on conversions of raw and torrefied woody pellet biomass to biofuels based on our previous findings.
The main purpose of this study was to investigate the feasibility of using biochar as a low cost catalyst in raw and torrefied biomass microwave pyrolysis. The effects of loadings of the biochar catalysts on product yields were determined. The characterization of the products from the biomass catalytic pyrolysis using biochar catalyst was evaluated and the effects of biochar catalyst and possible reaction mechanisms determined. The products from raw and torrefied biomass microwave catalytic pyrolysis were compared, and the recycling of biochar catalysts and their thermal behaviors were discussed. In addition, bio-oil upgrading under biochar catalyst was studied.
2. Materials and methods
2.1 Materials
Douglas fir sawdust pellets (DF) and torrefied Douglas fir sawdust pellets (TDF) were used in this study. The Douglas fir sawdust pellets were purchased from Bear Mountain Forest Products Inc. (USA) and their properties can be found in our previous paper.25 Torrefied Douglas fir sawdust pellets were prepared by a lab bench-scale microwave reactor at the reaction temperature of 275 °C for a total reaction time of 15 min with the power input of 600 W.2.2 Catalyst
Corn stover biochar produced from microwave pyrolysis were used as a catalyst. The corn stover (collected from Brookings, South Dakota, USA and dried in air at room temperature) was ground to 2 mm and pyrolyzed at the temperature of 650 °C with the power input of 700 W. The cold biochar catalyst was collected after microwave pyrolysis and used for subsequent catalysis experiments. Mineral analysis of the biochar catalyst was previously reported.142.3 Biomass catalytic pyrolysis
A lab-scale Sineo MAS-II batch microwave oven (Sineo Microwave Chemistry Technology Company, Shanghai, China) was used to conduct the biomass catalytic pyrolysis at the reaction temperature of 480 °C for 10 min with 700 W power setting. The ratios of biochar catalyst to biomass were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1
4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 1
2, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with a fixed biomass loading of 25 g. The Douglas fir pellets or torrefied pellets were first introduced in a half-liter quartz flask, and the corn stover biochar in a certain ratio to pellets was then added to the flask to cover the feedstock. The detailed experimental setting, including the nitrogen purging, temperature measurement, power input control, and condensers can be found in our previous papers.14,24,25 The catalyst and biochar formed from pellet biomass pyrolysis were separated according to their difference in particle sizes. All experiments were carried out in triplicate. The weight of syngas from biomass catalytic pyrolysis was calculated based on the weight difference using the following equation:
1 with a fixed biomass loading of 25 g. The Douglas fir pellets or torrefied pellets were first introduced in a half-liter quartz flask, and the corn stover biochar in a certain ratio to pellets was then added to the flask to cover the feedstock. The detailed experimental setting, including the nitrogen purging, temperature measurement, power input control, and condensers can be found in our previous papers.14,24,25 The catalyst and biochar formed from pellet biomass pyrolysis were separated according to their difference in particle sizes. All experiments were carried out in triplicate. The weight of syngas from biomass catalytic pyrolysis was calculated based on the weight difference using the following equation:| weight of syngas = initial pellet mass − bio-oil mass − pellet char mass | (1) |
2.4 Thermal behavior analysis of corn stover biochar by thermal gravimetric analyzer (TGA)
The thermal behavior of raw biochar catalyst and biochar catalysts after 1 and 10 recycles were analyzed by TGA (Mettler Toledo 188 TGA/SDTA 851, Switzerland). The TG analysis was performed using a nitrogen atmosphere with the flow rate of 20 ml min−1 and the temperature increased from 25 °C to 105 °C with a heating rate of 50 °C min−1, held for 10 min at 105 °C, increased to 800 °C with a heating rate of 20 °C min−1, and then held for 10 min at 800 °C.2.5 Crude bio-oil upgrading by biochar catalyst
Crude bio-oil from pine wood fludized bed pyrolysis was used in bio-oil upgrading over biochar catalyst. The same reactor as described in Section 2.3 were employed. The ratios of biochar catalyst to crude bio-oil were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 3
2 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The crude bio-oil was first introduced in a half-liter quartz flask, and the corn stover biochar in a certain ratio to crude bio-oil was then added to the flask to cover the crude bio-oil. The upgraded bio-oil was collected in a condensation system described in Section 2.3.
1. The crude bio-oil was first introduced in a half-liter quartz flask, and the corn stover biochar in a certain ratio to crude bio-oil was then added to the flask to cover the crude bio-oil. The upgraded bio-oil was collected in a condensation system described in Section 2.3.
2.6 GC/MS analysis for bio-oil
Chemical compositions of bio-oils were determined using an Agilent 7890A GC/MS (Agilent Technologies, USA) with a DB-5 capillary column. The detailed experimental analysis process was presented in Lei et al.142.7 GC analysis for syngas
Chemical compositions of syngas were determined by a Carle AGC 400 gas chromatography (GC) system with a thermal conductivity detector (TCD). The details of experimental settings can be found in Elliott et al.263. Results and discussion
3.1 Product yields
The biochar catalyst in biomass microwave pyrolysis has both functions of microwave receptor and catalyst to promote pyrolysis by increasing the heating rate and inducing self-gasification by forming “micro plasma”.27,28 In this study the average heating rate observed was about 100 °C min−1. The product yields had a close relationship with the loadings of the biochar catalyst (Fig. 1). The syngas yield ranged from 44.2 ± 1.25–46.1 ± 0.95 wt% in catalytic pyrolysis of raw Douglas fir pellets, and 41.4 ± 1.99–44.5 ± 1.96 wt% in catalytic pyrolysis of torrified Douglas fir pellets, both much higher than those reported in previous findings for the same pyrolysis conditions without catalyst.24,25 Hence, the biochar catalyst favored the syngas production as found in other study.27 The bio-oil yield was within the range of 25.8 ± 1.46–37.1 ± 0.80 wt% in catalytic pyrolysis of raw Douglas fir pellets, and 18.1 ± 1.09–25.0 ± 0.16 wt% in catalytic pyrolysis of torrefied Douglas fir pellets. The biochar yields were in the range of 18.7 ± 0.49–28.1 ± 2.4 wt% and 27.2 ± 1.80–40.5 ± 2.06 wt%, respectively, in catalytic pyrolysis of raw and torrefied Douglas fir pellets. Both bio-oil and biochar yields were reduced compared to those from pyrolysis without catalysts. In this study, catalytic pyrolysis of torrefied Douglas fir pellets produced less bio-oil and higher pellet biochar compared to the catalytic pyrolysis of raw Douglas fir pellets (Fig. 1). This phenomenon might be due to the carbonization of biomass in the torrefaction process.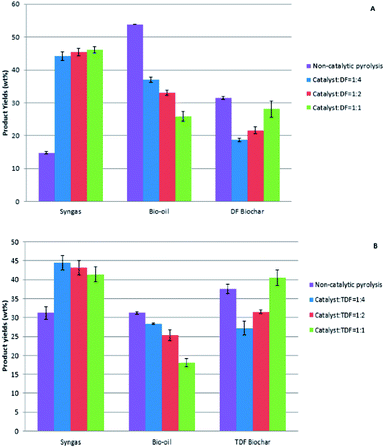 | ||
| Fig. 1 The effect of biochar catalyst loading on product yields from catalytic pyrolysis of (A): raw Douglas fir pellets (DF) and (B): torrefied Douglas fir pellets (TDF). | ||
The ratio of biochar catalyst to biomass significantly influenced the product yields, especially the bio-oil and biochar yields (Fig. 1). The bio-oil yield significantly decreased with the increase of the ratio of biochar catalyst to biomass in both raw and torrefied Douglas fir pellets catalytic pyrolysis but the DF biochar yields were increased. Syngas yield was about 41 wt% to 46 wt%, which was relatively stable under different ratios of biochar catalyst to biomass.
3.2 Bio-oil analysis by GC/MS
Fig. 2 shows the chemical distribution in bio-oils based on the functional groups. The compositions of bio-oils were greatly influenced by the biochar catalyst and its loadings. Few organic acids were detected in the bio-oils from both the raw and torrefied Douglas fir pellets catalytic pyrolysis over biochar catalyst. Phenols and guaiacols were significantly changed compared to those from the biomass pyrolysis without catalyst in previous studies.24 The phenols were 30 area% to 38 area% and slightly increased with the increase of the biochar catalyst loading in raw Douglas fir pellets catalytic pyrolysis while the guaiacols slightly decreased. The same trends were observed at the low loadings of the biochar catalyst in torrefied Douglas fir pellets catalytic pyrolysis. However, at high loadings of the biochar catalyst (ratio of biochar catalyst to biomass = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the phenols and guaiacols were 46 and 14 area%, respectively, indicating that most guaiacols were converted to phenols. Few hydrocarbons were found in Douglas fir pellets pyrolysis without catalyst or with activated carbon (AC) catalyst from previous studies.24,29 In this study, the hydrocarbons were about 4.25 area% to 8.31 area% and increased with the increase of biochar catalyst loading in catalytic pyrolysis of raw Douglas fir pellets. The hydrocarbons were also increased in catalytic pyrolysis of torrefied Douglas fir pellets with the increase of biochar catalyst loadings. The amount of hydrocarbons at low biochar catalyst loadings was close to that from torrefied Douglas fir pellets pyrolysis without catalysts. The yield of hydrocarbons at the biochar catalyst to biomass ratio of 1
1), the phenols and guaiacols were 46 and 14 area%, respectively, indicating that most guaiacols were converted to phenols. Few hydrocarbons were found in Douglas fir pellets pyrolysis without catalyst or with activated carbon (AC) catalyst from previous studies.24,29 In this study, the hydrocarbons were about 4.25 area% to 8.31 area% and increased with the increase of biochar catalyst loading in catalytic pyrolysis of raw Douglas fir pellets. The hydrocarbons were also increased in catalytic pyrolysis of torrefied Douglas fir pellets with the increase of biochar catalyst loadings. The amount of hydrocarbons at low biochar catalyst loadings was close to that from torrefied Douglas fir pellets pyrolysis without catalysts. The yield of hydrocarbons at the biochar catalyst to biomass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (up to 15.61 area%) was much higher than the yields from torrefied biomass pyrolysis and catalytic pyrolysis of the raw Douglas fir pellets. These results indicated that the loading of biochar catalyst is an important factor affecting the bio-oil composition and hydrocarbons' production. Further, it was easier for the torrefied biomass, compared to the raw biomass, to be converted to phenols and hydrocarbons under biochar catalysts.
1 (up to 15.61 area%) was much higher than the yields from torrefied biomass pyrolysis and catalytic pyrolysis of the raw Douglas fir pellets. These results indicated that the loading of biochar catalyst is an important factor affecting the bio-oil composition and hydrocarbons' production. Further, it was easier for the torrefied biomass, compared to the raw biomass, to be converted to phenols and hydrocarbons under biochar catalysts.
 | ||
| Fig. 2 Chemical distribution of bio-oil from raw (DF) and torrefied (TDF) Douglas fir pellets pyrolysis under different biochar catalyst loadings. | ||
Since the biochar catalyst was placed on top of the biomass, the volatiles from biomass decomposition first passed through the biochar zone prior to condensation. Within this catalysis zone, the cracking and reforming of the volatiles possibly occurred, involving heterogeneous solid–gas reactions and gas–gas reactions. One reaction mechanism could be the guaiacols cracking by the cleavage of methyl from O–CH3 to phenols over biochar catalysts. This explanation was supported by our observations that the guaiacols decreased while the phenols increased with the increase of catalyst loadings. However, the concentration of hydrocarbons increased with the increased biochar catalyst loading and more than 15 area% was obtained at the high catalyst loadings. The hydrocarbons might have been produced from both phenolics and aliphatic compounds as they were all noticeably reduced.
3.3 Syngas analysis by GC
The compositions of syngas and their variations under different biochar catalyst loadings are shown in Fig. 3. The syngas were mainly composed of CO, H2, CO2, CH4, and short-chain hydrocarbons, such as C2H4, C2H6, and C3H8. CO was the predominant chemical in the syngas, which first increased and then decreased with the increase of biochar catalyst loading in both raw and torrefied biomass catalytic pyrolysis. The highest concentrations of CO were 43.03 and 38.34 vol%, respectively, detected at the biochar catalyst to biomass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in both raw and torrefied biomass catalytic pyrolysis. The methane contents were significantly increased from about 1.5–7.7 vol% from Douglas fir pellets pyrolysis without catalyst24 to about 10.2–18.5 vol% in syngas by biochar catalysis.
2 in both raw and torrefied biomass catalytic pyrolysis. The methane contents were significantly increased from about 1.5–7.7 vol% from Douglas fir pellets pyrolysis without catalyst24 to about 10.2–18.5 vol% in syngas by biochar catalysis.
 | ||
| Fig. 3 The effect of biochar catalyst loading on the chemical composition of syngas from catalytic pyrolysis of (A): raw Douglas fir pellets (DF) and (B): torrefied Douglas fir pellets (TDF). | ||
In previous research no hydrogen was observed in the Douglas fir pellets pyrolysis without catalyst.24 But in raw Douglas fir pellets catalytic pyrolysis over biochar catalysts about 11.86 vol% to 20.43 vol% hydrogen was observed, and 21.36 vol% to 27.02 vol% hydrogen was also obtained in torrefied pellets catalytic pyrolysis over biochar catalysts, much higher than those in torrefied Douglas fir pellet pyrolysis without catalyst. These results indicated that biochar as a catalyst favored the hydrogen production as also found by Menendez et al.27 In contrast, the concentration of hydrogen decreased first and then increased with the increase of biochar catalyst loadings. The relatively low amount of CO2, less than 25 vol%, was produced in this study. CO2 from catalytic pyrolysis of raw Douglas fir pellets was increased with the increase of biochar catalyst loading. In comparison, the concentration of CO2 from catalytic pyrolysis of torrefied Douglas fir pellets was stable at the biochar catalyst to biomass ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The concentrations of CH4 in syngas from catalytic pyrolysis of raw and torrefied biomass were higher than 10 vol% and decreased with the increase of biochar catalyst loadings.
1. The concentrations of CH4 in syngas from catalytic pyrolysis of raw and torrefied biomass were higher than 10 vol% and decreased with the increase of biochar catalyst loadings.
Mominguez et al. reported that CH4 and CO2 can be reformed to syngas under biochar catalyst by microwave heating.27 This mechanism can partly explain our findings that the CH4 decreased and hydrogen increased with the increase of biochar catalyst loadings. However, the concentration of CO2 increased with the increase of biochar catalyst loading in this study (Fig. 3). The deoxygenation of bio-oils might have contributed to the CO2 production.
Noticeable differences in the concentrations of syngas components between raw and torrefied Douglas fir pellets catalytic pyrolysis were observed. More CO and CO2 were produced in the former process than in the latter; however, the H2 production in the latter was increased by up to 27.02 vol% at the biochar catalyst to biomass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
3.4 Thermal gravimetric (TG) analysis for biochar catalyst
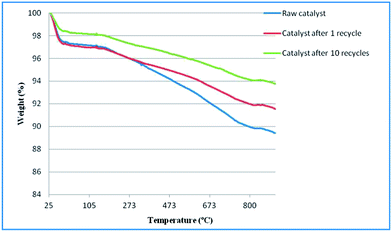
The thermal behavior of corn stover raw biochar and biochar catalysts after 1 and 10 recycles in Douglas fir pellets catalytic pyrolysis were analyzed by TGA under N2 flow of 20 ml min−1. The TG curves of these three samples are shown in Fig. 4. The small weight loss observed at the temperature below 150 °C was about 2–3% for the raw and recycled biochars. This weight loss was mainly due to evaporation of water and low molecular volatiles. At the temperature over 150 °C, there was a continuous, slight weight loss in raw and recycled biochar catalysts. A larger weight loss of the raw biochar catalyst occurred at the temperatures above 300 °C, suggesting the presence of volatiles in the raw biochar, detectable even at the high temperature of 650 °C. Still, the total weight loss in these three samples was only 4% to 6% at the temperature from 150 to 800 °C, indicating the stability of both the raw and recycled biochars.In biomass catalytic pyrolysis coke deposits on the catalyst are generally aromatic compounds and they can be removed at the temperatures over 350 °C.30 In this study, the weight loss from temperature 350 °C to 800 °C was only 2–3 wt% in recycled biochar and there was no significant weight loss change among different recycled biochar catalysts. It might be due to low coke deposits on the biochar catalyst in microwave assisted process and this result was also consistent with the findings that the weight of biochar did not change after the Pyrolysis.31
3.5 Crude bio-oil upgrading using biochar as catalyst
The upgraded bio-oil and syngas yields was about 41 wt% and 37 wt% at the catalyst to crude bio-oil ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and about 24 wt% and 73 wt% at the catalyst to crude bio-oil ratio of 3
2 and about 24 wt% and 73 wt% at the catalyst to crude bio-oil ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. In this study we also observed the cokes deposited in the biochar catalyst which was about 22 wt% and 4 wt% for catalyst loading at 1
1. In this study we also observed the cokes deposited in the biochar catalyst which was about 22 wt% and 4 wt% for catalyst loading at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 3
2 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 respectively. These results indicate that the high biochar loading favors the syngas production and reduces the cokes formation. The chemical compositions of upgraded bio-oil using biochar as catalyst were analyzed using GC/MS. Fig. 5 reveals the changes of main chemical groups with different biochar catalyst loadings. The concentrations of hydrocarbons and phenols were increased with the increase of biochar catalyst loading while those of the guaiacols and aliphatic compounds decreased. The hydrocarbons and phenols were up to 42.56 area% and 37.23 area%, respectively, at the biochar to bio-oil ratio of 3
1 respectively. These results indicate that the high biochar loading favors the syngas production and reduces the cokes formation. The chemical compositions of upgraded bio-oil using biochar as catalyst were analyzed using GC/MS. Fig. 5 reveals the changes of main chemical groups with different biochar catalyst loadings. The concentrations of hydrocarbons and phenols were increased with the increase of biochar catalyst loading while those of the guaiacols and aliphatic compounds decreased. The hydrocarbons and phenols were up to 42.56 area% and 37.23 area%, respectively, at the biochar to bio-oil ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Meanwhile, the concentrations of guaiacols and aliphatic compounds were reduced from about 40 area% to 6.11 area% and 13.76 area%, respectively. Hence, the biochar as catalyst facilitated the upgrading of the crude bio-oil to phenols and hydrocarbons.
1. Meanwhile, the concentrations of guaiacols and aliphatic compounds were reduced from about 40 area% to 6.11 area% and 13.76 area%, respectively. Hence, the biochar as catalyst facilitated the upgrading of the crude bio-oil to phenols and hydrocarbons.
3.6 Mechanism analysis of biomass catalytic pyrolysis and bio-oil upgrading over biochar catalysts
The reaction mechanism of biomass catalytic pyrolysis and bio-oil upgrading for hydrocarbons and syngas production using biochar catalysts is shown in Fig. 6. Both aliphatic compounds from pyrolysis of cellulose and hemicellulose and guaiacols from lignin decomposition contributed to the aromatics production. The reaction pathway of glucose catalytic pyrolysis to aromatics proposed by Carlson et al. can be used to explain the aromatic hydrocarbon production from aliphatic compounds.32 The intermediate chemicals mainly composed of anhydrosugars and furans which were first formed from hemicelluloses and cellulose decomposition. The carboxylic acid sites presented on the biochar surface33 catalyzed the anhydrosugars dehydration to furans which were converted to aromatics by oligomerization, decarboxylation and decarbonylation. This was confirmed by observed increase of water content in the bio-oil with biochar catalyst pyrolysis (water content in the bio-oil increased from about 40 wt% and 29 wt% to 49–63 wt% and 43–59 wt% for raw and torrefied biomass pyrolysis respectively). Aromatics were also converted from lignin. The lignin predominantly decomposed to guaiacols, and then guaiacols were cracked to phenols by cleavage of bond O–CH3. This was confirmed by significantly increased methane content (from about 1.5–7.7 vol% from Douglas fir pellets pyrolysis without biochar catalyst24 to about 10.2–18.5 vol% in syngas by biochar catalysis) in syngas. The phenols were further cracked to aromatics by the cleavage of –OH. The biochar catalyst with carboxylic functional groups on its surface promoted the cracking and upgrading of bio-oils. | ||
| Fig. 6 Reaction pathway of biomass catalytic pyrolysis and bio-oil upgrading for hydrocarbon and syngas production using biochar catalyst under microwave heating. | ||
In this study, the syngas with high concentrations of H2 and CO was obtained and two main reaction mechanisms can reveal the H2 and CO production. The syngas from Douglas fir pyrolysis without biochar catalyst was mainly composed of CO (64 vol%), CH4 (1.5–7.7 vol%), CO2 (1.2 vol%), and short-chain hydrocarbons.24 The water contents of raw bio-oils ranged from 17.2 wt% to 24.7 wt% based on dry biomass. Water and CO entered biochar catalysts. When the water gas shift reaction is catalyzed by biochar catalysts, this resulted in an equivalent of hydrogen, which was confirmed by increased hydrogen content (from no hydrogen without using biochar catalyst24 to about 11.86–20.43 vol% hydrogen by Douglas fir pellets catalytic pyrolysis over biochar catalysts, and to 21.36–27.02 vol% hydrogen by torrefied pellets catalytic pyrolysis over biochar catalysts) and decreased CO content (from 64 vol% without using biochar catalyst24 to less than 43 vol% by biochar catalysis) in syngas after catalysis. This reaction might be triggered by the metals such as Cu and Fe presented in the biochar catalyst14,34 and enhanced by the microwave irradiation. Another reaction mechanism for H2 and CO production is dry reforming of methane. Dominguez et al.31 and Muradov et al.22 reported that the biochar catalyzed biogas conversion to syngas at 800 °C and both K present in biochar and microwave irradiation favored the biogas conversion. The observed decrease of methane with the increase of biochar catalyst loading in this study confirmed the occurrence of dry reforming of methane. It is worth to note that the gas produced in this study immediately went into the biochar catalyst. It means the reactions of methane production and conversion can be considered simultaneously which occurred at the temperature the same as that of bio-oil upgrading, which was much lower than regular gasification temperature (e.g. 800 °C). Dominguez et al.31 pointed out that the self-gasification of the char occurred in microwave pyrolysis and K presented in biochar favored this gasification and reduced the coke formation. This explained the low coking observed in the recycled biochar catalysts under this study.
4. Conclusions
The effects of biochar as a catalyst in biomass catalytic pyrolysis and bio-oil upgrading were investigated. The biochar catalyst favored the syngas production and had positive influence on the bio-oil quality. The bio-oil chemical profile from catalytic pyrolysis and bio-oil upgrading over biochar catalysts was simplified to phenols and hydrocarbons, and their concentrations were increased with the increase of biochar catalyst loadings. High-quality syngas richened in H2, CO, and CH4 was obtained for biomass catalytic pyrolysis over biochar catalysts. These results indicated that biochar might be a cheap catalyst in biomass conversion and bio-oil upgrading.Acknowledgements
This work was supported in part by the Pacific Northwest National Laboratory and the the Joint Center for Aerospace and Technology Innovation (JCATI). The authors thank the scientists of Pacific Northwest National Laboratory (PNNL), Alan Zacher and Todd Hart, for their contributions and assistance in syngas analysis.References
- D. S. Scott and J. Piskorz, Can. J. Chem. Eng., 1984, 62, 404–412 CrossRef CAS.
- D. Mohan, C. U. Pittman and P. H. Steele, Energy Fuels, 2006, 20, 848–889 CrossRef CAS.
- D. C. Elliott, T. R. Hart, G. G. Neuenschwander, L. J. Rotness and A. H. Zacher, Environ. Prog. Sustainable Energy, 2009, 28, 441–449 CrossRef CAS.
- M. M. Wright, D. E. Daugaardc, J. A. Satriob and R. C. Brown, Fuel, 2010, 89, S2–S10 CrossRef CAS PubMed.
- M. Ikura, M. Stanciulescu and E. Hogan, Biomass Bioenergy, 2003, 24, 221–232 CrossRef CAS.
- R. N. Hilten, B. P. Bibens, J. R. Kastner and K. C. Das, Energy Fuels, 2010, 24, 673–682 CrossRef CAS.
- C. Rioche, S. Kulkarni, F. C. Meunier, J. P. Breen and R. Burch, Appl. Catal., B, 2005, 61, 130–139 CrossRef CAS PubMed.
- Z. Wang, Y. Pan, T. Dong, X. Zhu, T. Kan, L. Yuan, Y. Torimoto, M. Sadakata and Q. Li, Appl. Catal., A, 2007, 320, 24–34 CrossRef CAS PubMed.
- A. A. Lappas, M. C. Samolada, D. K. Iatridis, S. S. Voutetakis and I. A. Vasalos, Fuel, 2002, 81, 2087–2095 CrossRef CAS.
- I. Demiral and S. Sensoz, Bioresour. Technol., 2008, 99, 8002–8007 CrossRef CAS PubMed.
- M. C. Samolada, A. Papafotica and I. A. Vasalos, Energy Fuels, 2000, 14, 1161–1167 CrossRef CAS.
- H. Zhang, R. Xiao, H. Huang, D. Wang, Z. Zhong, M. Song, Q. Pan and G. He, Energy Fuels, 2009, 23, 6199–6206 CrossRef CAS.
- Y. Chun, G. Sheng, C. T. Chiou and B. Xing, Environ. Sci. Technol., 2004, 38, 4649–4655 CrossRef CAS.
- H. Lei, S. Ren and J. Julson, Energy Fuels, 2009, 23, 3254–3261 CrossRef CAS.
- C. A. Mullen, A. A. Boateng, N. M. Goldberg, I. M. Lima, D. A. Laird and K. B. Hicks, Biomass Bioenergy, 2010, 34, 67–74 CrossRef CAS PubMed.
- K. Y. Chan, L. Van Zwieten, I. Meszaros, A. Downie and S. Joseph, Aust. J. Soil Res., 2007, 45, 629–634 CrossRef CAS.
- D. Mohan, R. Sharma, V. K. Singh, P. Steele and C. U. Jr Pittman, Ind. Eng. Chem. Res., 2012, 51, 900–914 CrossRef CAS.
- R. Azargohar and A. K. Dalai, Appl. Biochem. Biotechnol., 2006, 129–132 Search PubMed.
- A. M. Dehkhoda, A. H. West and N. Ellis, Appl. Catal., A, 2010, 382, 197–204 CrossRef CAS PubMed.
- G. Chen and B. Fang, Bioresour. Technol., 2011, 102, 2635–2640 CrossRef CAS PubMed.
- J. R. Kastner, J. Miller, D. P. Geller, J. Locklin, L. H. Keith and T. Johnson, Catal. Today, 2012, 190, 122–132 CrossRef CAS PubMed.
- N. Muradov, B. Fidalgo, A. C. Gujar, N. Garceau and A. T-Raissi, Biomass Bioenergy, 2012, 42, 123–131 CrossRef CAS PubMed.
- R. Ormsby, J. R. Kastner and J. Miller, Catal. Today, 2012, 190, 89–97 CrossRef CAS PubMed.
- S. Ren, H. Lei, L. Wang, Q. Bu, S. Chen, J. Wu, J. Julson and R. Ruan, J. Anal. Appl. Pyrolysis, 2012, 94, 163–169 CrossRef CAS PubMed.
- S. Ren, H. Lei, L. Wang, Q. Bu, S. Chen, J. Wu, J. Julson and R. Ruan, Bioresour. Technol., 2013, 135, 659–664 CrossRef CAS PubMed.
- D. C. Elliott, T. R. Hart and G. G. Neuenschwander, Ind. Eng. Chem. Res., 2006, 45, 3776–3781 CrossRef CAS.
- J. A. Menendez, A. Dominguez, Y. Fernandez and J. J. Pis, Energy Fuels, 2007, 21, 373–378 CrossRef CAS.
- A. A. Salema and F. N. Ani, Bioresour. Technol., 2011, 102, 3388–3395 CrossRef CAS PubMed.
- Q. Bu, H. Lei, S. Ren, L. Wang, Q. Zhang, J. Tang and R. Ruan, Bioresour. Technol., 2012, 108, 274–279 CrossRef CAS PubMed.
- X. Guo, Y. Zheng, B. Zhang and J. Chen, Biomass Bioenergy, 2009, 33, 1469–1473 CrossRef CAS PubMed.
- A. Dominguez, Y. Fernandez, B. Fidalgo, J. J. Pis and J. A. Menendez, Energy Fuels, 2007, 21, 2066–2071 CrossRef CAS.
- T. R. Carlson, J. Jae, Y. Lin, G. A. Tompsett and G. W. Huber, J. Catal., 2010, 270, 110–124 CrossRef CAS PubMed.
- J. E. Amonette and S. Joseph, Characteristics of Biochar: Microchemical Properties, in Biochar for Environmental Management, ed. J. Lehmann and S. Joseph, Earthscan, London, 2009, pp. 33–52 Search PubMed.
- W. H. Chen, J. G. Jheng and A. B. Yu, Int. J. Hydrogen Energy, 2008, 33, 4789–4797 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |