Bio-functionalized Pt nanoparticles based electrochemical impedance immunosensor for human cardiac myoglobin
Sujeet K. Mishraab,
Avanish K. Srivastavaa,
Devendra Kumarb and
Rajesh*a
aCSIR-National Physical Laboratory, Dr K. S. Krishnan Road, New Delhi-110012, India. E-mail: rajesh_csir@yahoo.com
bDepartment of Applied Chemistry, Delhi Technological University, Bawana Road, Delhi-110042, India
First published on 17th April 2014
Abstract
We report the covalent immobilization of three-dimensional carboxyl-functionalized Pt(MPA) nanoparticles with myoglobin protein antibody by carbodiimide coupling reaction deposited onto an indium-tin-oxide-coated glass plate for the construction of a bioelectrode. This bioelectrode assembly was characterized by spectro/microscopic and electrochemical techniques. Electrochemical impedance studies of the bioelectrode showed significant changes in charge transfer resistance (Ret), predominantly in the low AC frequency region of <40 Hz, on immunoreaction with human cardiac myoglobin antigen (Ag-cMb). Ag-cMb detection in phosphate buffer exhibited a linear range of 0.01 μg mL−1 to 1 μg mL−1 with a sensitivity of 184.8 Ω cm2 per decade.
1. Introduction
Nanostructured materials such as nanoparticles, nanotubes, and nano hybrid materials have become an intensive research area due to their unique chemical and physical properties including large specific surface areas, good biocompatibilities, and high surface free energies.1 Recent advances in the field of nanotechnology have opened up research opportunities on materials with ultrafine nanostructures. Metal nanoparticles, with their fascinating properties such as large surface-to-volume ratios and increased surface activities, have continued to draw a great deal of attention in interdisciplinary areas of the scientific community. Metal nanoparticles have been used in intriguing applications in many fields of physical, chemical and material sciences in the past few years.2–4 Recently, the amount of research on their biological applications has increased, with potential applications in the construction of electrochemical sensors and biosensors, where they function as “electron antennae” to efficiently channel electrons between the electrode and the electroactive species.5,6 Functionalized nanomaterials offer excellent prospects for interfacing biological recognition events with electronic signal transduction, leading to the design of a new generation of bioelectronic devices that exhibit novel functions. The major advantage of using functionalized nanomaterials is their potential capacity for combining multiple modalities within a single probe, which enables far higher sensitivities to be achieved. Platinum (Pt), one of the most researched noble metals, has extensive applications in the field of electrochemical sensors. This is because Pt nanoparticles can act as an effective matrix for biomolecules due to their biocompatibility and large surface areas.7 Recent applications of Pt nanoparticles include the detection of low concentrations of DNA by using a platinum nanoparticle–gold ultra microelectrode in a hydrazine oxidation reaction.8According to a WHO report (2011), cardiovascular diseases (CVDs) are considered to be the leading cause of death around the world.9 CVDs include coronary heart disease, cerebrovascular disease, raised blood pressure, peripheral artery disease, rheumatic heart disease and congenital heart disease and heart failure. Cardiac markers are biological analytes that are detectable in the blood serum and play an essential role in the diagnosis, prognosis, monitoring, and risk stratification in suspected CVD patients.10 Among the currently-used cardiac markers for the detection of acute myocardial infarction (AMI),11 myoglobin (found in heart and skeletal muscles) has been proven to be a valuable screening test for early AMI diagnosis. Myoglobin concentration (cMb) in blood rises quickly, i.e. 1–3 h, after the appearance of the initial symptoms of the disease, and reaches its maximum concentration between 6 and 12 h before returning to baseline within 24–48 h. Myoglobin is a 17.6 kDa monomeric hemeprotein that contains 153 amino acid residues in a highly-folded and compact structure with eight separate and distinct alpha helical secondary structures. Due to its small molecular dimensions (3 nm × 4 nm × 5 nm) and molar volume (1.87 × 104 cm3 mol−1),12 it is quickly released into circulation, making cMb a valuable early screening test for AMI. The normal cMb level in human blood ranges from 30 to 90 ng mL−1, which spikes to 200 ng mL−1 or even higher within 1 h of the onset of myocardial infarction. cMB can reach as high as 900 ng mL−1 during the peak hour. In general, tests for detecting cMb are either indicative rapid tests or estimations using sandwich immunoassay with secondary-labelled antibodies, i.e. the enzyme linked immunosorbent assay (ELISA) technique. However, these tests require the proper laboratory equipment and instrumentation, multi-step processing of samples, and well-trained personnel, leading to a time-consuming and expensive detection. Masson et al. developed a surface plasmon resonance (SPR)-based sensor for myoglobin.13 This type of detection is relatively easy and cheap to perform, and also allows quantitative and kinetic measurements of molecular interactions. The main problems associated with the SPR sensor are the fouling ability, low affinity and specificity that affect sensitivity of biosensing transducers. Other types of optical biosensors used for Mb detection include those developed by Darain et al.14 and Matveeva et al.15 These sensors can perform enhanced Mb immunoassays, however, they are expensive and require dedicated personnel to perform the tests. They also require difficult labelling procedures that depend on indirect indicator-based signal schemes. Matveeva et al. have utilized silver island films (SIF) for developing a fluorescence-based immunoassay for cardiac Mb. The main disadvantage of this method is the non-homogeneous nature of SIFs, which results in significant deviations in assay readings, making this method unsuitable for precise Mb detection.15
Electrochemical immunosensors are an important class of sensing systems that have revolutionized modern chemical analysis due to their easy use, high efficiency, possibility of portability and miniaturization, fast response time and direct transduction of bio-molecular recognition events into electronic signals. Because of their high accuracy, sensitivity, selectivity and cost-effectiveness, they have been extensively used to detect proteins, biomarkers, biological toxins and biological-warfare agents in critical situation, food, environment, pharmaceutical chemistry, and clinical diagnostic applications.16–20 Pakapongpan et al.21 have reported an electrochemical sensor for Mb based on a methylene blue-multiwalled carbon nanotubes (MWCNT) nanohybrid modified glassy carbon electrode (GCE), where the detection is based on the direct electrochemical reduction of Mb. Although this sensor provided a wide linear range of Mb detection from 0.1 μM to 3.0 μM (∼1.78 μg mL−1 to 53.40 μg mL−1), it is out of the physiological range of cMb in human blood, and thus would require a sample dilution for low level, sub-μg mL−1 Mb detection.
Electrochemical impedance spectroscopy (EIS) has recently received considerable attention for providing a sensitive and non-destructive characterization of the electrical properties in biological interfaces such as sensing formation of antigen–antibody and biotin–avidin complexes and interactions between oligonucleotides and DNAs.22,23
In this paper, considering the advantages of the EIS method and the properties of metal Pt nanoparticles, we report a platform for the construction of a bioelectrode for the detection of the cardiac biomarker, cMb, using functionalized Pt nanoparticles. The functionalized Pt nanoparticles are covalently anchored on a 3-aminopropyltriethoxy silane (APTES) self assembled monolayer (SAM) over an indium-tin-oxide (ITO)-glass plate. The cardiac protein antibody, Ab-cMb, is covalently attached to the carboxyl-functionalized Pt nanoparticle-modified APTES/ITO-glass plates using a carbodiimide coupling reaction. This bioelectrode is systematically characterized by various microscopic techniques, and its immunosensing ability towards the quantitative estimation of Ag-cMb in phosphate buffer saline (PBS; pH 7.4) is investigated by EIS using [Fe (CN)6]3−/4− as a redox probe.
2. Experimental
2.1 Reagents
Monoclonal mouse anti-human cardiac myoglobin (Ab-cMb; Cat 4M23) and myoglobin derived from human heart tissue (Ag-cMb; Cat 8M50) were procured from Hytest (Turku, Finland). Mouse immunoglobulin-G (Ag-IgG; Cat IGP3) were obtained from GENEI (Bangalore, India). 3-Aminopropyl triethoxysilane (APTES) was purchased from Merck chemicals (Germany). N-(3-Dimethyl aminopropyl)-N′-ethyl carbodiimide hydro chloride (EDC), N-hydroxy succinimide 98% (NHS), hydrogen hexachloroplatinate hexahydrate (H2PtCl6·6H2O), sodium borohydride (NaBH4) and 3-mercaptopropionic acid (MPA) were obtained from Sigma-Aldrich Corp. All other chemicals were of analytical grade and used without further purification.2.2 Apparatus
Contact angles were recorded on a model DSA10MK2 Drop Shape Analysis System from Krüss GmbH, Germany. XRD patterns were recorded using a Bruker AXS Advance D8 powder X-ray diffractometer. High resolution transmission electron microscopy was performed on a Tecnai G2 F30 STWIN HRTEM model with field emission gun operated at 300 kV. Scanning electron microscopy (SEM) images were obtained with a LEO 440 PC (UK) digital scanning electron micrograph with a mounted energy dispersive X-ray spectrometer (EDX). Fourier-transform infrared (FTIR) spectrum was taken on a Perkin-Elmer Spectrum BX II. Atomic force microscopy (AFM) images were obtained on a Nanoscope 5 (VEECO Instrument Ltd., USA). Cyclic voltammetry and EIS measurements were done on a PGSTAT302N AUTOLAB instrument from Eco Chemie, Netherlands. The EIS experimental data were circuit fitted by GPES (general purpose electrochemical system version 4.9, Eco Chemie) software to obtain the values of EIS parameters.2.3 Synthesis of functionalized Pt nanoparticles
3-Mercaptopropionic acid-capped Pt (MPA) nanoparticles were chemically synthesized using the conventional sodium borohydride reduction method in aqueous solution as reported earlier, with slight modification.24 Briefly, a 100 mM stock solution of Pt salt was prepared by dissolving 1 g of H2PtCl6·6H2O in 19 mL of deionized water. 1 mL of this stock solution was then reduced by adding NaBH4 solution (48 mg in 2 mL H2O) drop wise under constant stirring. The change in colour of the solution from yellow to dark brown indicated the formation of the Pt nanoparticles. A solution of 46 μL MPA in 10 mL H2O was then immediately added to stabilize the above Pt colloidal solution. The final volume of the reaction mixture was kept at 50 mL. The reaction mixture was left stirring for 2 h at room temperature. Thereafter, the colloidal solution was washed 2 to 3 times with ethanol, centrifuged at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, and vacuum dried for 12 h.
000 rpm, and vacuum dried for 12 h.
2.4 Preparation of bioelectrode
The ITO coated glass plates (10 Ω □−1) were sequentially cleaned by ultrasonic cleaning in extran, acetone, ethanol, isopropyl alcohol and DI water for 10 min each followed by drying in vacuum. The cleaned ITO glass plates were then exposed to oxygen plasma for 5 min in a plasma chamber to increase the amount of hydroxyl groups present on the ITO-glass surface. After this, the ITO-glass plates were immersed in 2% APTES solution prepared in ethanol for 1.5 h under ambient conditions to form a SAM of APTES. These were subsequently rinsed with ethanol in order to remove non-bonded APTES from the surface of the substrate and dried under N2 gas flow. The carboxyl groups present on the Pt(MPA) nanoparticles were activated to prepare amine-reactive esters of carboxylate groups by adding 30 mM NHS and 150 mM EDC to a 5 mL aqueous solution of 0.1 mg mL−1 Pt(MPA) nanoparticles. The APTES-modified ITO glass plates were then immersed in the above solution mixture of activated functionalized Pt(MPA) nanoparticles for 3 h, followed by washing with double-distilled water and drying under N2 gas flow to obtain Pt (MPA-NHS)/APTES/ITO-glass. Ab-cMb was immobilized onto the above modified electrode by treating it overnight with PBS (pH 7.4) containing 100 μg mL−1 Ab-cMb at 4 °C, followed by washing with PBS and drying under N2 flow. The Ab-cMb-immobilized electrode was then incubated in 1% BSA solution for 30 min to block the nonspecific binding sites on the electrode surface, washed with PBS to remove any physically adsorbed antibodies and dried under N2 flow. The stepwise fabrication of the prototype assembly of the Ab-cMb/Pt(MPA)/APTES/ITO-glass electrode is schematically represented in Scheme 1.2.5 EIS measurement procedure
All electrochemical measurements were carried out in a conventional three-electrode cell configuration consisting of the proposed modified electrode as the working electrode, Ag/AgCl as a reference electrode and a platinum wire as a counter electrode. Electrochemical impedance spectroscopy was conducted in PBS (pH 7.4, 0.1 M KCl) solution containing 2 mM [Fe(CN)6]3−/[Fe(CN)6]4− in the frequency range from 1 Hz to 100 kHz at an AC voltage of 0.05 V. From a stock solution of 100 μg mL−1 Ag-cMb, aliquots of different concentrations were prepared in PBS. The EIS response of the Ab-cMb/Pt(MPA)/APTES/ITO-glass bioelectrode was measured after the addition of successive aliquots of different concentrations of Ag-cMb in PBS solution containing 2 mM [Fe(CN)6]3−/[Fe(CN)6]4−. An impedance measurement was conducted with a sample solution containing no Ag-cMb, and the corresponding electron transfer resistance (Ret) observed in a Nyquist plot was taken as the control sample response. Subsequently, Ret and other related EIS parameters were measured after the successive addition of aliquots of different Ag-cMb concentrations to detect the antibody–antigen immunoreaction.3. Results and discussion
3.1 Contact angle measurement
Contact angle measurement based on the sessile drop method was used to determine the hydrophobic/hydrophilic character of the electrode surface using water droplets as the test liquid probe. This method offers an easy-to-measure indication of the nature of the functional groups present on the uppermost surface layers of the modified electrode surface. Water droplets were manually introduced onto the electrode surface with a micro-syringe, and digital snapshots were taken of the droplets on the surface and analysed with software provided by Kruss. Four replicates for each measurement at different steps of modification were taken to establish statistical significance. The bare ITO-glass electrode with surface hydroxyl groups shows hydrophilic behaviour with a contact angle (θ) of 40 ± 1° (Fig. 1a). The surface polarity is altered after the modification of the ITO-glass electrode by hydrophobic alkyl chains of APTES molecules. This modification results in silanization, which decreases the surface energy by drastically reducing the number of hydroxyl groups on the bare ITO-glass; the resulting θ increases to 78 ± 1° (Fig. 1b). The covalent attachment of Pt(MPA) nanoparticles on APTES/ITO-glass leads to the formation of a hydrophilic surface comprised of carboxyl groups, which decreases θ to 59 ± 2° (Fig. 1c). After immobilization of the protein antibody, θ increases significantly to 91 ± 1° (Fig. 1d) due to the replacement of hydrophilic carboxyl groups by the hydrophobic amino acid chains of protein antibody molecules. This indicates the attachment of Ab-cMb molecules on the Pt(MPA)-modified electrode surface assembly. | ||
| Fig. 1 Contact angle measurement images of (a) ITO coated glass plate; (b) APTES/ITO-glass; (c) Pt(MPA)/APTES/ITO-glass; and (d) Ab-cMb/Pt(MPA)/APTES/ITO-glass. | ||
3.2 Microstructural characteristics
XRD was used to examine the possible crystallinity of the Pt nanoparticles formed after sodium borohydride reduction. Fig. 2 shows the XRD pattern of the functionalized Pt nanoparticles. The sample exhibits broad characteristic diffraction peaks of Pt with face center-cubic (f.c.c.) structure (JCPDS, card no. 4-802). The strongest diffraction peak was located at a 2θ of 39.46° (111), with other two strong peaks at 45.88° (200) and 67.32° (220). The existence of broad intense peaks in the X-ray spectrum indicates the presence of Pt nanoparticles with nanocrystalline dimensions. Since the breadth remains almost consistent for each peak, the broadening is certainly due to crystallite size. By using the XRD peaks, the average size of the Pt nanoparticles can be estimated by the width of the reflection according to the Debye–Scherrer equation (eqn (1)):
D = 0.9λ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ
| (1) |
The average particle size of the Pt nanoparticles was calculated to be approximately 5.14 nm.
Pt nanoparticle size was further confirmed by employing high resolution transmission electron microscopy. In general, the microstructure was uniform with individual particles revealing facetted growth, sharp edges and a tendency to intermingle with each other (Fig. 3a). At the atomic scale, it is obvious that there is good interfacing between the particles. Four such nanoparticles are clearly marked as A to D in Fig. 3b. In most instances, these particles are overlapped and abut each other. A set of moiré fringes are also discerned (region marked as dotted circle in Fig. 3b). It is important to mention that at the atomic scale, the individual particles are interpreted as nano-crystallites with stacking of atomic planes with particular crystallographic orientations. The interplanar spacings of three such planes are 0.23, 0.20 and 0.14 nm, which corresponding to hkl 111, 200 and 220, respectively, of the Pt face centered cubic structure (space group: Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, and lattice parameter: a = 0.39 nm, ref. JCPDS card no. 040802); these are marked on the micrograph (Fig. 3b). The measurements carried out in Fig. 3a and b further delineated the average size of these nanoparticles as between 4 and 5 nm (Fig. 3c), however, these particles generally ranged between 1.5 and 8.5 nm on the Gaussian graph.
m, and lattice parameter: a = 0.39 nm, ref. JCPDS card no. 040802); these are marked on the micrograph (Fig. 3b). The measurements carried out in Fig. 3a and b further delineated the average size of these nanoparticles as between 4 and 5 nm (Fig. 3c), however, these particles generally ranged between 1.5 and 8.5 nm on the Gaussian graph.
Fig. 4 shows the typical EDX pattern of Pt-NP-functionalized APTES-modified ITO-glass with an inset showing the SEM image of the corresponding electrode area. The EDX spectra demonstrate the presence of Pt-NPs, along with other elements of the corresponding silane and ITO-glass. The SEM image shown in Fig. 4 is one of the three electrodes prepared simultaneously. The SEM image shows metallic granular particles of Pt nanoparticles distributed over the modified electrode surface area.
 | ||
| Fig. 4 EDX spectra of Pt(MPA) nanoparticles on ITO and inset shows corresponding SEM area at the magnification of 50 KX. | ||
Fig. 5 shows FTIR spectra of the (a) APTES/ITO (b) Pt(MPA)/APTES/ITO and (c) Ab-cMb/Pt(MPA)/APTES/ITO taken in attenuated total reflection mode. For APTES (Fig. 5a), the spectrum shows the Si–O–Si characteristic band at 1055 cm−1.25 The characteristic peak observed at 1750 cm−1 (Fig. 5b) is due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations of the carboxylic groups of the MPA-functionalized Pt nanoparticles. The peaks at 2965 cm−1 and 936 cm−1 correspond to the –OH stretching and bending vibrations of the carboxylic acid group. After the immobilization of Ab-cMb, additional peaks at 3387 cm−1 and 1612 cm−1 were observed (Fig. 5c), representing the N–H stretching and bending vibrations,26 respectively. These are indicative of amide bond formation between the Pt(MPA) nanoparticles and Ab-cMb molecules.
O stretching vibrations of the carboxylic groups of the MPA-functionalized Pt nanoparticles. The peaks at 2965 cm−1 and 936 cm−1 correspond to the –OH stretching and bending vibrations of the carboxylic acid group. After the immobilization of Ab-cMb, additional peaks at 3387 cm−1 and 1612 cm−1 were observed (Fig. 5c), representing the N–H stretching and bending vibrations,26 respectively. These are indicative of amide bond formation between the Pt(MPA) nanoparticles and Ab-cMb molecules.
 | ||
| Fig. 5 FTIR spectra of (a) APTES/ITO-glass (b) Pt(MPA)/APTES/ITO-glass and (c) Ab-cMb/Pt(MPA)/APTES/ITO-glass. | ||
The surface morphology of each modification step involved in the fabrication of the Pt(MPA)-modified bioelectrode was characterized using AFM images taken in non-contact mode (Fig. 6).
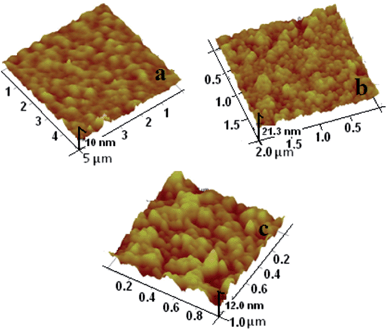 | ||
| Fig. 6 AFM images of (a) APTES/ITO-glass (b) Pt(MPA)/APTES/ITO-glass and (c) Ab-cMb/Pt(MPA)/APTES/ITO-glass. | ||
The surface roughness parameter (Ra) and root mean square roughness (Rq) are the two amplitude parameters that were used to study the temporal changes occurring during the creation of a new surface. They were also used to determine spatial differences when studying the surface features at different scales in terms of irregularity and height distribution. Fig. 6a shows the AFM image of the SAM of APTES on an ITO-glass surface, which has a regular island-like structure with only a few surface aggregates. The corresponding Ra and Rq of the APTES/ITO-glass surface are 0.886 nm and 1.08 nm, respectively. The surface morphology changes to a dense and complete one with granular particles spreading all over it on modification with Pt(MPA) nanoparticles (Fig. 6b). The values of Ra and Rq are increased to 1.56 nm and 1.95 nm, respectively. However, the AFM image (Fig. 6c) of the bioelectrode shows a completely changed morphology upon Ab-cMb immobilization, with an appreciable increase in Ra to 1.72 nm and Rq to 2.12 nm. This morphology is much bigger and more globular than that seen in Pt(MPA)/APTES/ITO-glass, which is a characteristic of natural protein.27 The significant increase in both the Ra and Rq values indicated the immobilization of the Ab-cMb molecules on the surface of the Pt-NP-modified electrode.
3.3 Electrochemical characterization of the bioelectrode
EIS has been chosen as the main characterization technique to assess the immunosensor performance since it is known to be an effective tool for the qualitative and quantitative characterization of electrochemical processes occurring at the electrode/solution interface of modified electrodes. EIS monitors the response of the studied system to the application of a small amplitude AC signal at different frequencies. Although this technique does not lead to the identification of the chemical bonds or intermediates, vital information regarding the reaction rates occurring at the electrode/solution interface can be obtained. This is usually done using an ‘equivalent circuit’ comprised of an assembly of electrical circuit elements that model the physicoelectric characteristics of the electrode/solution interface. In the present work, the experimentally-fitted Randles equivalent circuit model was employed, which includes the following four elements: (i) the ohmic resistance of the electrolyte solution, Rs; (ii) the Warburg impedance, Zw, resulting from the diffusion of ions from the bulk electrolyte to the electrode interface; (iii) the interfacial double layer capacitance (Cdl) between an electrode and a solution, related to the surface condition of the electrode and (iv) the electron-transfer resistance, Ret. This equivalent circuit model has been modified by a constant phase element (CPE) that replaces the classical capacitance; this allows the incorporation of the Helmholtz double layer and the electrode surface roughness or heterogeneity. The CPE is also in a parallel arrangement with both Ret and Zw, all of which are in a series arrangement with Rs. The impedance related to CPE is given by eqn (2):| ZCPE(ω) = 1/Y0(jω)n | (2) |
| Type of electrodes | Ret (Ω cm2) | CPE | Zw (× 10−3) (Ω cm2) | χ2 (× 10−4) | δ (μm) | |
|---|---|---|---|---|---|---|
| Y0 (μF cm−2) | n | |||||
| a Ret = charge transfer resistance; CPE = constant phase element; δ = diffusion layer thickness; Zw = Warburg resistance. | ||||||
| ITO-glass | 85.7 | 2.76 | 0.93 | 3.45 | 1.88 | 55.44 |
| APTES/ITO | 25.2 | 4.82 | 0.87 | 3.42 | 1.13 | 49.69 |
| Pt(MPA)/APTES/ITO | 107.0 | 3.07 | 0.88 | 3.67 | 1.99 | 61.68 |
| Pt/APTES/ITO | 9.0 | 13.19 | 0.77 | 3.22 | 1.38 | 47.09 |
| Ab-cMb/Pt(MPA)/APTES/ITO | 183.5 | 2.44 | 0.96 | 3.90 | 2.30 | 72.74 |
A more clear description of the frequency-dependent behaviour of different circuit elements of the fitted Randles circuit is provided by the Bode plots, as shown in Fig. 7b. Valuable information regarding certain kinetic phenomena occurring at the electrode/solution interface at different ranges of applied frequencies can be obtained from these plots. The plots show that at the very low frequency region of <10 Hz, a diffusive nature is observed for the APTES/ITO-glass electrode. The diffusive nature is somewhat reduced on modification by Pt(MPA) nanoparticles, and finally disappears on immobilization of Ab-cMb on the modified electrode. The dominance of Ret behaviour in the low frequency region for the bioelectrode reflects its biocompatible nature, even at such low frequency.
Davies and Compton proposed a term known as the diffusion layer thickness δ, which helps in categorizing the type of diffusion occurring at the modified electrodes. The value of δ can be obtained from the modified Einstein equation for the root mean square displacement of diffusing particles,28 as shown in eqn (3) and listed in Table 1:
| δ = (2DΔE/ν)1/2 | (3) |
3.4 Surface coverage and nature of the bioelectrode
The fractional coverage area (θ) of the functionalized Pt-NPs over the silane SAM can be calculated from the impedance diagram using eqn (4):29| θ = 1 − Ret1/Ret2 | (4) |
The type of electrochemical mechanism occurring at the electrode/electrolyte interface of the Ab-cMb/Pt (MPA)/APTES/ITO-glass bioelectrode can be obtained from the relationship between the peak current and the scan rate. Fig. 8 shows the cyclic voltammogram (CV) of the resulting bioelectrode in 2 mM [Fe(CN)6]3−/4− at different scan rates ranging from 25–125 mV s−1. A plot of the anodic (Ipa) and cathodic peak currents (Ipc) versus the square root of the scan rates (ν1/2) are shown as an inset in Fig. 8.
 | ||
| Fig. 8 CV of the bioelectrode as a function of scan rate in PBS (pH 7.4, 0.1 M KCl) containing 2 mM [Fe(CN)6]3−/4−. Inset: plot of peak current vs. ν1/2 (mV s−1)1/2. | ||
A linear relationship with good correlation between the peak currents and the square root of scan rate was obtained, suggesting a diffusion-controlled process at the bioelectrode/solution interface. The linear regression line obtained for the relationship between the anodic peak current Ipa and ν1/2 can be expressed by eqn (5),
| Ipa(ν1/2) = bν1/2 + c | (5) |
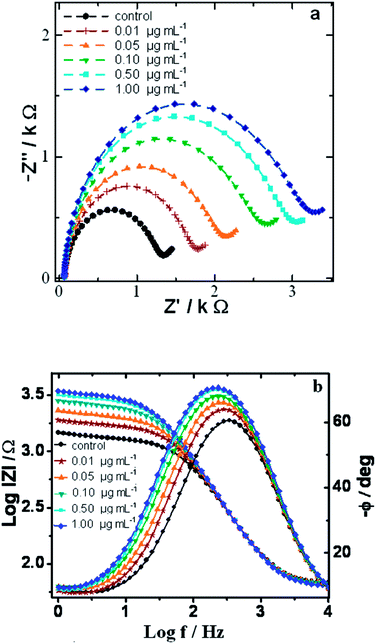
3.5 Electrochemical impedance response of the bioelectrode towards protein antigen Ag-cMb
The specific immunoreaction of Ab-cMb to its complimentary target Ag-cMb at the electrode surface results in the formation of an antibody–antigen complex. The formation of an immunocomplex results in the creation of a kinetic barrier, which perturbs the interfacial electron transport at the bioelectrode/solution interface. As the Faradaic reaction of a redox couple becomes increasingly hindered, the electron transfer resistance increases, and the capacitance decreases accordingly. The sample solution without a target protein, Ag-cMb, was taken as the control sample, and the corresponding Ret value was taken as the control sample response. Fig. 9a shows the Nyquist plots obtained after the addition of successive aliquots of different concentrations of target protein antigen, and the corresponding fitting values of Ret are given in Table 2. The plot shows a noticeable increase in the diameter of the Nyquist circles with increasing concentration of added Ag-cMb due to antigen–antibody interaction. A small but noticeable decrease in Y0 was also observed, indicating a decrease in the capacitive behaviour of the bioelectrode with immunoreaction.| Concentration of Ag-cMb | Ret (Ω cm2) | CPE | Zw (× 10−3) (Ω cm2) | χ2 (× 10−4) | |
|---|---|---|---|---|---|
| Y0 (μF cm−2) | n | ||||
| Control | 183.5 | 2.44 | 0.960 | 3.90 | 2.30 |
| 0.01 μg mL−1 | 288.3 | 2.26 | 0.970 | 2.20 | 2.10 |
| 0.05 μg mL−1 | 406.5 | 2.18 | 0.974 | 1.37 | 2.01 |
| 0.10 μg mL−1 | 494.3 | 2.15 | 0.976 | 1.02 | 2.74 |
| 0.50 μg mL−1 | 601.5 | 2.13 | 0.974 | 0.92 | 2.67 |
| 1.00 μg mL−1 | 647.8 | 2.08 | 0.979 | 0.82 | 3.12 |
The corresponding Bode frequency spectrum upon the above immunoreactions occurring at the electrode surface is shown in Fig. 9b. In the high frequency region of f > 4 kHz, the total impedance, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |Z|, remains unchanged with a phase angle (φ) of ∼0°, indicating that electrolyte solution resistance, Rs, was unchanged. In the intermediate frequency range from 4 kHz to 40 Hz, an almost straight line curve was obtained with 55° < φ < 90°, indicating pseudo-capacitance behaviour of the bioelectrode. Negligible changes with the addition of target Ag-cMb in the sample solution were observed in this region, which indicated that the proposed bioelectrode cannot function as a capacitive immunosensor. In the low frequency region (f < 40 Hz), visible changes were observed in the impedance modulus lines upon immunoreactions with increasing concentrations of added target Ag-cMb. As this region is dominated by Ret, this confirmed our choice of using changes in Ret as the main sensing element.
|Z|, remains unchanged with a phase angle (φ) of ∼0°, indicating that electrolyte solution resistance, Rs, was unchanged. In the intermediate frequency range from 4 kHz to 40 Hz, an almost straight line curve was obtained with 55° < φ < 90°, indicating pseudo-capacitance behaviour of the bioelectrode. Negligible changes with the addition of target Ag-cMb in the sample solution were observed in this region, which indicated that the proposed bioelectrode cannot function as a capacitive immunosensor. In the low frequency region (f < 40 Hz), visible changes were observed in the impedance modulus lines upon immunoreactions with increasing concentrations of added target Ag-cMb. As this region is dominated by Ret, this confirmed our choice of using changes in Ret as the main sensing element.
The sensitivity of the as-prepared bioelectrode was obtained by plotting (Fig. 10) the change in specific electron charge transfer resistance (ΔRet = (Ret)after immunoreaction − (Ret)control) vs. the logarithmic value of Ag-cMb concentration in the range of 10 ng mL−1 to 1 μg mL−1.
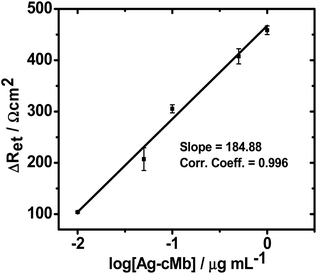 | ||
| Fig. 10 Concentration dependent calibration curve of the bioelectrode. The error bars represent the standard deviation from three separate experiments. | ||
Eqn (6) depicts the obtained linear relationship, where the slope of the line is the sensitivity of the bioelectrode:
ΔRet(log[Ag-cMb]) = b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log[Ag-cMb] + d log[Ag-cMb] + d
| (6) |
The Ret sensitivity (slope b of the calibration curve) of the bioelectrode was found to be 184.8 Ω cm2 per decade of Ag-cMb with intercept d = 473.74 Ω cm2. The correlation regression coefficient was 0.996 (n = 5). The limit of detection, calculated as three times the signal-to-noise ratio, was found to be 1.70 ng mL−1. The EIS experimental procedure requires about 12 min for the complete assay, including 3 min for sample preparation without any pretreatment (compared to a few days for ELISA), showing its potential usefulness for the early detection of AMI. These results show that this bioelectrode is competitive with the other recently reported semiconductor/metal nanoparticle/carbon nanomaterials and polymer based Mb sensors15,21,30 in terms of both physiological range for cMb detection with minimum sample preparation time and experimental steps and high sensitivity. A comparative analytical performance of some electrochemical-based systems for Mb detection is given in Table 3. The high electroactive nature of the Pt(MPA)-NPs makes this bioelectrode more sensitive to Mb detection than the recently reported ZnS NPs-based bioelectrode, which exhibited a comparatively low sensitivity of 117.36 Ω cm2 per decade.31
| Electrochemical sensor type | Transduction platform | Linear range | Limit of detection (LOD) | References |
|---|---|---|---|---|
| Flow injection analysis with amperometry | MB-MWNTs/GCE | 1.78 μg mL−1 to 53.4 μg mL−1 | 353.98 ng mL−1 | 21 |
| Impedimetric | SAM/Au electrode | 10−12 to 10−6 M | 15 ng mL−1 | 35 |
| Potentiometric | SAM of alkane thiol/gold-coated silicon chip | — | 1000 ng mL−1 | 34 |
| Square wave voltammetry | SPE/MeNP-DDAB/anti-cMb | 10 ng mL−1 to 400 ng mL−1 | 5 ng mL−1 | 30 |
| Cyclic voltammetry | TiO2 nanotubes/GCE | 1 μg mL−1 to 100 μg mL−1 | 1000 ng mL−1 | 33 |
| Impedimetric | MUA-MPA/Au-wire | 10 ng mL−1 to 650 ng mL−1 | — | 32 |
| Impedimetric | Pt(MPA)/APTES/ITO | 10 ng mL−1 to 1000 ng mL−1 | 1.7 ng mL−1 | Present work |
The reproducibility of the bioelectrode was evaluated by measuring the Ret response for each added concentration of the target Ag-cMb for three different bioelectrodes prepared independently under similar experimental conditions. The inter-assay variation coefficient was found to be within the range of 1.5–18% at an individual Ag-cMb concentration, indicating an acceptable precision and fabrication reproducibility. The stability of the bioelectrode was examined by repeatedly carrying out the impedance measurements on the bioelectrode for the same concentration of target Ag-cMb under identical conditions. Even after five repeated impedance measurements, no appreciable changes in the impedimetric response were observed, revealing that the bioelectrode retains its biocompatible property both in solution and in the open. A sustained Ret response towards a fixed Ag-cMb concentration was obtained with the individual bioelectrode stored at 4 °C for at least two months, indicating good shelf-life stability. The specificity of the bioelectrode towards Ag-cMb was tested by carrying out the immunoreaction with non-specific mouse IgG in the range of 10 ng mL−1 to 1 μg mL−1 under identical conditions. No significant changes were observed in Ret with the added aliquots of IgG with respect to the control sample. This may be attributed to either the absence of an antigen–antibody interaction, or a weak non-specific interaction, confirming the specificity of this bioelectrode.
4. Conclusions
This work demonstrates a detailed EIS characterization of a bioelectrode based on functionalized Pt-NPs for the investigation of bioaffinity interaction towards the detection of the prognostic cardiac marker, cMb. The functionalized Pt-NPs with large surface-to-volume ratios and free –COOH linkage groups remarkably improved the properties of the bioelectrode in terms of stability and sensitivity. Both the microstructural and electrochemical characteristics of the bioelectrode have been extensively characterized by AFM, TEM, XRD, EDX, and EIS techniques. The dominance of Ret behaviour in the low frequency region of the EIS spectra of the bioelectrode demonstrated its biocompatible nature. The bioelectrode shows a small capacitance change with a high resistive component of impedance, showing the specific and sensitive relationship to the electron transfer rate, and thereby providing a direct response to antibody–antigen interaction on immunoreaction. High protein loading with efficient covalent bonding to the Pt-NPs results in the construction of a bioelectrode with a wide linear detection range, good sensitivity, biocompatibility and acceptable reproducibility. The protocol devised here is simple, efficient and inexpensive. After optimization with blood serum, it can also be used as a model for establishing general methods for the detection and quantitative analysis of other protein marker assays in the fields of clinical diagnostics and molecular biology.Acknowledgements
We are grateful to Prof. R. C. Budhani, Director, National Physical Laboratory, New Delhi, India for providing the facilities. S. K. Mishra is thankful to the Council of Scientific and Industrial Research, India for providing a senior research fellowship (SRF).References
- G. Hodes, Adv. Mater., 2007, 19, 639 CrossRef CAS.
- M. Adaris, L. Marzo, J. Pons, D. A. Blake and A. Merkoçi, Biosens. Bioelectron., 2013, 47, 190 CrossRef PubMed.
- W. Wu, M. Wu, Z. Sun, G. Li, Y. Ma, X. Liu, X. Wang and X. Chen, J. Alloys Compd., 2013, 579, 117 CrossRef CAS PubMed.
- Y. Chen, Y. Tang, S. Luo, C. Liu and Y. Li, J. Alloys Compd., 2013, 578, 242 CrossRef CAS PubMed.
- X. Xu, X. Liu, Y. Li and Y. Ying, Biosens. Bioelectron., 2013, 47, 361 CrossRef CAS PubMed.
- Q. Kang, L. Yang and Q. Cai, Bioelectrochemistry, 2008, 74, 62 CrossRef CAS PubMed.
- J. Wang, D. F. Thomas and A. Chen, Anal. Chem., 2008, 80, 997 CrossRef CAS PubMed.
- S. J. Kwon and A. J. Bard, J. Am. Chem. Soc., 2012, 134, 10777 CrossRef CAS PubMed.
- T. Kong, R. Su, B. Zhang, Q. Zhang and G. Cheng, Biosens. Bioelectron., 2012, 34, 267 CrossRef CAS PubMed.
- A. Qureshi, Y. Gurbuz and J. H. Niazi, Sens. Actuators, B, 2012, 171, 62 CrossRef PubMed.
- B. McDonnel, S. Hearty, P. Leonard and R. O'Kennedy, Clin. Biochem., 2009, 42, 549 CrossRef PubMed.
- N. S. K. Gunda and S. K. Mitra, Biomicrofluidics, 2010, 4, 014105 CrossRef PubMed.
- J. F. Masson, L. Obando, S. Beaudoin and K. Booksh, Talanta, 2004, 62, 865 CrossRef CAS PubMed.
- F. Darain, P. Yager, K. L. Gan and S. C. Tjin, Biosens. Bioelectron., 2009, 24, 1744 CrossRef CAS PubMed.
- E. G. Matveeva, Z. Gryczynski and J. R. Lakowicz, J. Immunol. Methods, 2005, 302, 26 CrossRef CAS PubMed.
- L. Bonel, J. C. Vidal, P. Duato and J. R. Castillo, Anal. Methods, 2010, 2, 335 RSC.
- B. Jeong, R. Akter, O. H. Han, C. K. Rhee and M. A. Rahman, Anal. Chem., 2013, 85, 1784 CrossRef CAS PubMed.
- J. Li, L. T. Xiao, G. M. Zeng, G. H. Huang, G. L. Shen and R. Q. Yu, J. Agric. Food Chem., 2005, 53, 1348 CrossRef CAS PubMed.
- S. K. Arya, T. S. Pui, C. C. Wong, S. Kumar and A. R. A. Rahman, Langmuir, 2013, 29, 6770 CrossRef CAS PubMed.
- Q. Wei, Y. Zhao, B. Du, D. Wu, H. Li and M. Yang, Food Chem., 2012, 134, 1601 CrossRef CAS PubMed.
- S. Pakapongpan, R. Palangsuntikul and W. Surareungchai, Electrochim. Acta, 2011, 56, 683 CrossRef PubMed.
- A. Venkatanarayanan, T. E. Keyes and R. J. Forster, Anal. Chem., 2013, 85, 2216 CrossRef CAS PubMed.
- S. Shrikrishnan, K. Sankaran and V. Lakshminarayanan, J. Phys. Chem. C, 2012, 116, 16030 CAS.
- Y. Teow and S. Valiyaveettil, Nanoscale, 2010, 2, 2607 RSC.
- E. T. Vandenberg, L. Bertilsson, B. Liedberg, K. Uvdal, R. Erlandsson, H. Elwing and I. Lundstrom, J. Colloid Interface Sci., 1991, 147, 103 CrossRef CAS.
- A. Barth, Biochim. Biophys. Acta, 2007, 1767, 1073 CrossRef CAS PubMed.
- L. Yang and Y. Li, Biosens. Bioelectron., 2005, 20, 1407 CrossRef CAS PubMed.
- S. A. Mamuru and K. I. Ozoemena, Electroanalysis, 2010, 22, 985 CrossRef CAS.
- V. Ganesh, S. K. Pal, S. Kumar and V. Lakshminarayanan, J. Colloid Interface Sci., 2006, 296, 195 CrossRef CAS PubMed.
- E. V. Suprun, A. L. Shilovskaya, A. V. Lisitsa, T. V. Bulko, V. V. Shumyantseva and A. I. Archakov, Electroanalysis, 2011, 23, 1051 CrossRef CAS.
- S. K. Mishra, D. Kumar, A. M. Biradar and Rajesh, Bioelectrochemistry, 2012, 88, 118 CrossRef CAS PubMed.
- Rajesh, V. Sharma, V. K. Tanwar, S. K. Mishra and A. M. Biradar, Thin Solid Films, 2010, 519, 1167 CrossRef CAS PubMed.
- S. S. Mandal, K. K. Narayan and A. J. Bhattacharyya, J. Mater. Chem. B, 2013, 1, 3051 RSC.
- Y. Wang, Y. Zhou, J. Sokolov, B. Rigas, K. Levon and M. Rafailovich, Biosens. Bioelectron., 2008, 24, 162 CrossRef CAS PubMed.
- M. Billah, H. C. W. Hays and P. A. Millner, Microchim. Acta, 2008, 160, 447 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |