A novel approach to prepare polymer mixed-brushes via single crystal surface patterning†
S. Abbaspoor,
F. Abbasi* and
S. Agbolaghi
Institute of Polymeric Materials, Sahand University of Technology, Tabriz, Iran. E-mail: f.abbasi@sut.ac.ir
First published on 12th March 2014
Abstract
Polymer mixed-brushes with predetermined morphologies as well as with uniform chain length and chain length distribution of various brushes were prepared on a substrate via single crystal surface patterning with a self-seeding procedure from a dilute solution. The required materials including poly(ethylene glycol)-b-polystyrene (PEG-b-PS) and poly(ethylene glycol)-b-poly(methyl methacrylate) (PEG-b-PMMA) with the same crystalline block molecular weight and different amorphous block molecular weights were synthesized via atom transfer radical polymerization (ATRP). Based on various qualities of the employed solvent and various interactions of the substrate with amorphous blocks, the matrix (PS)-dispersed (PMMA) morphologies were obtained on PEG single crystals. The matrix and dispersed phase regions were detectable by atomic force microscopy (AFM) due to differences in height and stiffness. The domain size of dispersed phase decreased with an increase in the molecular weights of PS and PMMA blocks because hindrance against the presence of PEG-b-PMMA chains increased. Despite the domain size of the dispersed phase ranging from 286 to 351 nm, the crystallization temperature did not have a significant effect on the domain size. By increasing the crystallization temperature, the thickness enhancement, and consequently the tethering density enhancement, was more considerable for PS regions, which can be attributed to the lower osmotic pressure of PS chains. Finally, we grew epitaxial structures consisting of PEG-b-PS, PEG-b-PMMA, and homo-PEG layers to verify the determined thicknesses from mixed-brush systems through the conjunction thickness of the corresponding copolymer and homopolymer crystals.
Introduction
In order to use materials in various applications in industry, nanotechnology, and biomedicine, their surface properties should be modified.1–5 Various methods including mechanical techniques, monolayer adsorption, plasma treatment, chemical coatings, and the use of polymer chains have been applied to modify the surfaces.3,4,6,7 Due to the high sensitivity of polymers to the small environmental variations, polymer brushes are considered to be the best candidates for applying surface modifications.4,8–16 This property provides an opportunity for usage in many applications including biomaterials, microfluidic devices, tissue engineering, membrane surface modification, colloidal stabilization, chemical sensors, and ion-exchange adsorbents.9,17–28 To obtain a combination of various properties for a surface-grafted monolayer, the polymer mixed-brushes could be fabricated. Because different polymer brushes have various behaviours in mixed-brush structures, they are capable of introducing a wide range of morphologies as well as responses.9,20,29,30 To construct the polymer mixed-brush surface morphologies, various methods such as grafting to and from star-block copolymers and single crystal growth have previously been presented.9,20,30–37 The single crystal growth method, which is able to produce a homogeneous brush length and length distribution and can adjust accurate grafting density, has priority over other methods.37–40In our novel approach, mixed-brush single crystals were grown from a dilute solution including two amorphous-crystalline diblock copolymer chains with the same crystalline block. Our surface patterning was random, resembling that of leopard skin. We had access to true control of poly(methyl methacrylate) (PMMA)-dispersed domain (playing the role of spread patches) size in the PS-matrix, but the population of the PMMA domains was not predictable on a given surface area of a single crystal. In this work, we have employed the materials [poly(ethylene glycol)-b-polystyrene (PEG-b-PS)/poly(ethylene glycol)-b-poly(methyl methacrylate) (PEG-b-PMMA)] with a weight ratio of 50/50. Furthermore, the lateral habit of grown single crystals was investigated elsewhere.41
Here, the main reason for selecting the PS and PMMA amorphous blocks was their different compatibilities with the PEG crystalline block. Upon constructing the PEG substrate, although its interaction with PMMA brushes is attractive, the PS-tethered chains are repulsed from the substrate surface.40 The other reason is associated with the quality of applied solvent at growth conditions. Although at adopted conditions, amyl acetate is a very good solvent for PS blocks, it is a partially poor solvent for PMMA blocks,39,42–44 which is why we were able to design the matrix-dispersed surface morphologies during the growth of single crystals from the aforementioned diblock copolymer chains. This method could be referred to as a type of single crystal surface patterning. In this leopard skin-like pattern, patches are constructed of PMMA brushes, which are reluctant to abandon the PEG substrate surface, and the skin itself is formed of stretched PS-tethered chains. We used bulk atactic densities of PMMA and PS to perform our calculations.44,45 It is noteworthy that the constructed mixed-brush surface morphologies did not require detection of a selective solvent. As PMMA and PS amorphous blocks have different hardness values,38,46,47 the detection of phase regions on a single crystal substrate was possible.
Fabrication of epitaxial nanostructures from various amorphous-crystalline diblock copolymers including PEG-b-PS, PEG-b-PMMA, and homo-PEG chains was the last issue tackled in this work. In addition to having chemical and geometric recognition applications,42 channel-wire arrays have been employed in this work to verify the corresponding thicknesses of substrates in different phase regions of single crystals with mixed-brush surface morphologies. To grow the epitaxial structures, PEG5000-b-PS4600 single crystals, which exhibited the least hindrance against the growth of subsequent layers, were used as the innermost crystals. Through the growth of the homo-PEG layer, we obtained the sublayer thickness of the region covered by PS (![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) n = 4600 g mol−1) tethered chains by using the conjunction thickness between the PEG-b-PS and homo-PEG single crystal. Afterward, the PEG-b-PMMA and homo-PEG layers were grown to acquire the substrate thicknesses of surface areas covered by the PMMA-grafted chains via the corresponding conjunction thickness.
n = 4600 g mol−1) tethered chains by using the conjunction thickness between the PEG-b-PS and homo-PEG single crystal. Afterward, the PEG-b-PMMA and homo-PEG layers were grown to acquire the substrate thicknesses of surface areas covered by the PMMA-grafted chains via the corresponding conjunction thickness.
Experimental
Synthesis
The bromo-capped PEG macroinitiator (PEG-Br) (MI) was synthesized and purified according to literature.48,49 The diblock copolymers of PEG-b-PS and PEG-b-PMMA including various molecular weights of PS and PMMA blocks were synthesized via solution polymerization in chlorobenzene (CB, Merck, >99%) and with the agent ratio of ([styrene (St)]0/[MI]0/[CuBr]0/[bipy]0/[CB]0) = 240/1/1/3/66) and ([methyl methacrylate (MMA)]0/[MI]0/[CuCl]0[CuCl2]0/[bipy]0/[CB]0) = 177/1/1/0.07/3/375) by ATRP. The reaction temperatures for St and MMA polymerization were 110 and 65 °C, respectively. Due to the higher reactivity of MMA in comparison with St,50,51 CuCl2 (Merck, >98%) was used as a deactivator to better control the polymerization.52 Related explanations of synthesis procedures are described elsewhere.53,54 As characteristic for ATRP, the GPC traces (Fig. S1†) were narrow, symmetric, and mono-modal, and they moved with increasing conversion towards lower elution volume, i.e., elevated molecular weights. The molecular characterization data of diblock copolymers used in this work are reported in Table 1.![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) AMORPHn refers to the number-average molecular weight of PS or PMMA blocks)
AMORPHn refers to the number-average molecular weight of PS or PMMA blocks)
| Sample | ![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) n (g mol−1) n (g mol−1) |
![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) PEGn (g mol−1) PEGn (g mol−1) |
![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) AMORPHn (g mol−1) AMORPHn (g mol−1) |
![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) w/ w/![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) n n |
|---|---|---|---|---|
| Homo-PEG5000 | 5000 | 5000 | 0 | 1.06 |
| PEG5000-b-PS4600 | 9600 | 5000 | 4600 | 1.13 |
| PEG5000-b-PS10000 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
5000 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.15 |
| PEG5000-b-PS14800 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
5000 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.16 |
| PEG5000-b-PMMA8700 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
5000 | 8700 | 1.19 |
| PEG5000-b-PMMA13100 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
5000 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.21 |
| PEG5000-b-PMMA17100 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
5000 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.20 |
Single crystal growth
Details of the self-seeding technique used for growing PEG-b-PS diblock copolymer single crystals have been described elsewhere.55,56 Here, we have adopted this approach to grow single crystals with mixed-brush surface morphologies.Solution crystallization was conducted with a dilute concentration of 0.018 wt% (wt/wt of PEG-b-PS/PEG-b-PMMA was 50/50) in amyl acetate (Merck, >98%). The sample was put into a cell tube containing solvent. The tube was then sealed and heated to a temperature greater than that of the dissolution of the sample in the solvent (Td = 65 °C) in a temperature-controllable oil bath and was maintained for approximately 10–15 min to erase the thermal history and to reach a homogeneous solution. The cell tube was then transferred to another bath at −10 °C and kept for 5–6 h for fast crystallization and was then immersed into a given self-seeding temperature oil bath (Ts = 41 °C for all samples) and kept for 20 min. The cell tube was then quickly transferred into an isothermal oil bath at the desired crystallization temperature and was maintained for two to three days.
To fabricate the epitaxial structures, single crystals of diblock copolymers at a solute concentration of 0.009 wt% were grown in a similar manner. After complete crystallization of the diblock copolymers (or homopolymers), a few drops of the sample containing the copolymer (or homopolymer) single-crystal seeds were added to the homogeneous solution of the homopolymer (or copolymer) which had been kept in the same crystallization oil bath. In experiments of epitaxial growth, the exact amount of transferred seeds was not clear. However, the population changed from one sample to another. The various lateral sizes of channels proved our claim that wider channels relate to a lower transferred population of seeds. Nevertheless, in terms of volume, we transferred 0.7 ml of solution containing seeds in each stage. The homogeneous homopolymer (or copolymer) solution was prepared by heating the mixture of the homopolymer (or copolymer) and the solvent to 65 °C for 20 min. The solution was then quickly transferred to the crystallization oil bath (at a preset Tc) for approximately 10 min to reach thermal equilibrium before adding the diblock (or homopolymer) single-crystal seeds. During this period, no crystals were detected. The homopolymer (or copolymer) crystals then grew on the diblock copolymer (or homopolymer) single-crystal seeds to form composite single crystals.
Results and discussion
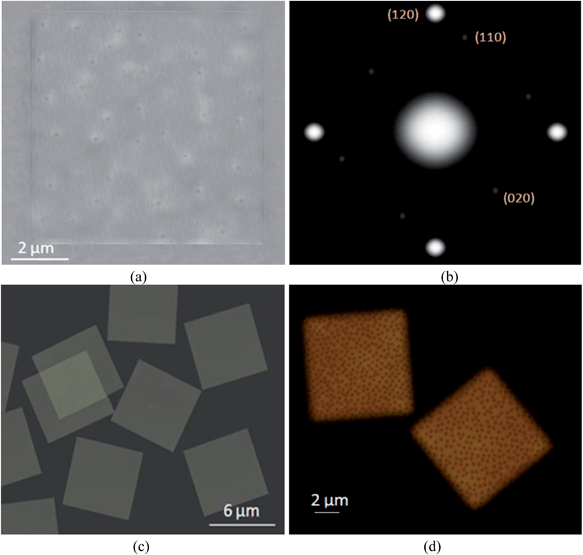
It has been found that PEG-b-PS diblock copolymers can crystallize in dilute solutions to grow single crystals having a PEG block single crystal as the middle layer and PS block layers at the top and bottom of the basal surfaces to give a sandwich structure.42,55,56 In this study, we have grown mixed-brush single crystals with matrix-dispersed surface morphology and square shapes. The strongest diffraction spots in Fig. 1(b) are ascribed to the (120), (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 20), (1
20), (1![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0), and (
0), and (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) planes (identical to homo-PEG57–60 and homo-brush single crystals39,42,43,60), showing that the PEG chain direction in the crystal is parallel to the surface normal. Fig. 1 depicts the transmission electron microscopy (TEM) bright image of a PEG5000-b-PS4600/PEG5000-b-PMMA8700 single crystal grown at Tc = 28 °C (a) and the related electron-diffraction (ED) pattern (b). Several darker spots in the TEM image illustrate the PMMA-dispersed patches with crystalline substrate thicknesses that differed from the respective substrate thickness of the PS-matrix. Fig. 1(c) shows the scanning transmission electron microscopy (STEM) image of a PEG5000-b-PS4600/PEG5000-b-PMMA13100 single crystal grown at Tc = 30 °C. It is apparent that all single crystals are identical; uniformity is a well-known feature of the self-seeding method.37,40
0) planes (identical to homo-PEG57–60 and homo-brush single crystals39,42,43,60), showing that the PEG chain direction in the crystal is parallel to the surface normal. Fig. 1 depicts the transmission electron microscopy (TEM) bright image of a PEG5000-b-PS4600/PEG5000-b-PMMA8700 single crystal grown at Tc = 28 °C (a) and the related electron-diffraction (ED) pattern (b). Several darker spots in the TEM image illustrate the PMMA-dispersed patches with crystalline substrate thicknesses that differed from the respective substrate thickness of the PS-matrix. Fig. 1(c) shows the scanning transmission electron microscopy (STEM) image of a PEG5000-b-PS4600/PEG5000-b-PMMA13100 single crystal grown at Tc = 30 °C. It is apparent that all single crystals are identical; uniformity is a well-known feature of the self-seeding method.37,40
Detection of various phase regions in mixed-brush morphologies
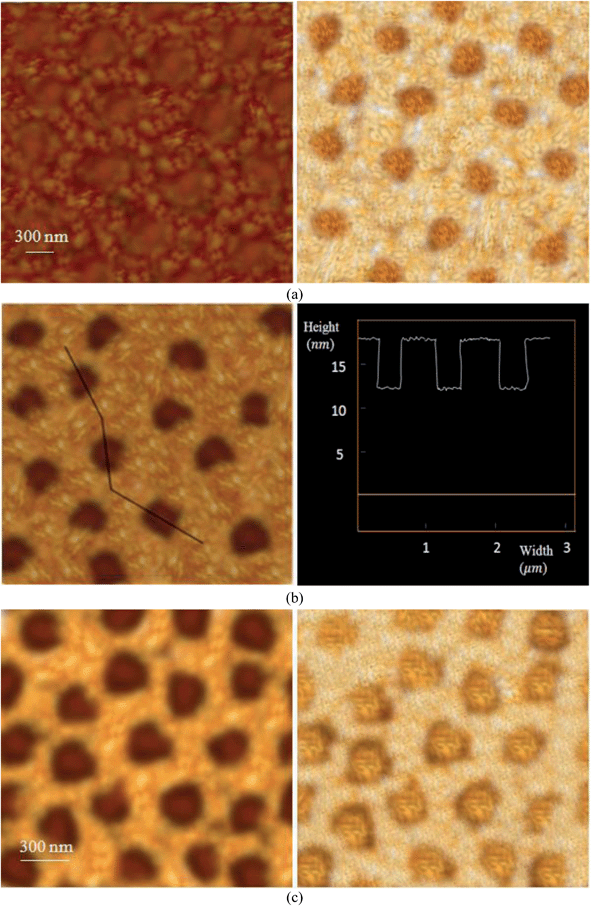
As mentioned previously, the mixed tethered chains on the substrate comprise two different phase areas, which fall into matrix and dispersed phases. Fig. 1(a) and (d) are representative of the TEM bright image of a PEG5000-b-PS4600/PEG5000-b-PMMA8700 single crystal grown at Tc = 28 °C and the AFM height image of a PEG5000-b-PS10000/PEG5000-b-PMMA13100 single crystal grown at Tc = 23 °C, respectively, which clearly indicate the mentioned morphology. Here, without the need for solvent treatments, we were able to distinguish the various matrix and dispersed phase regions. The ultimate ratio of PMMA-covered patches to PS-covered matrix region is less than 50/50 (primary ratio used to grow single crystals). This is to say that in growth systems, there have been occurrences of some single crystals covered only by PMMA brushes. Hence, the excess of PEG-b-PMMA diblock copolymers construct such homo-brush single crystals.Such a distribution for polymer mixed-brushes can be attributed to two main reasons. The first is the different conformations of PS and PMMA amorphous blocks in amyl acetate. The applied solvent at growth conditions of 23–32 °C could be considered a partially poor solvent for the PMMA block, whereas it is a very good solvent for the PS block.42–44 Therefore, the PS chains are more extended than those of PMMA. The mentioned conformation for PS blocks may cause PEG-b-PS chains to be included into the single-crystal structure more conveniently, which is why the surface areas composed of the PS-tethered chains construct the matrix phase. Second, as stated previously, the interaction of the substrate surface with PMMA grafted chains is attractive and that with PS grafted chains is repulsive. Hence, the PMMA grafted PMMA tend to be attracted to the substrate surface, which subsequently increases the segmental density in its vicinity. On the contrary, the PS chains are repulsed from the surface to reach more extended conformations. The PMMA chains possess a somewhat packed pancake shape conformation; therefore, their hindrance against the chains with the same blocks is significantly high. Due to compatibility of PMMA chains with PEG crystalline blocks61 and their more coiled conformations, the tethered PS chains treat them like homo-PEG chains, which enables these chains to enter the single crystal structure. Moreover, for high hindrance of PMMA grafted chains, the phase regions constructed with them could contribute only to small spread patches.
From a macroscopic perspective, PMMA is harder than PS on the basis of Rockwell hardness (M80–M105 for PMMA and M65–M85 for PS).38,46 From a microscopic perspective, the depth of a static indentation for an AFM tip penetrating PS regions is approximately 5 nm, whereas that penetrating PMMA domains is less than 1 nm.38,47 Therefore, the opaque phases are PMMA-dispersed.
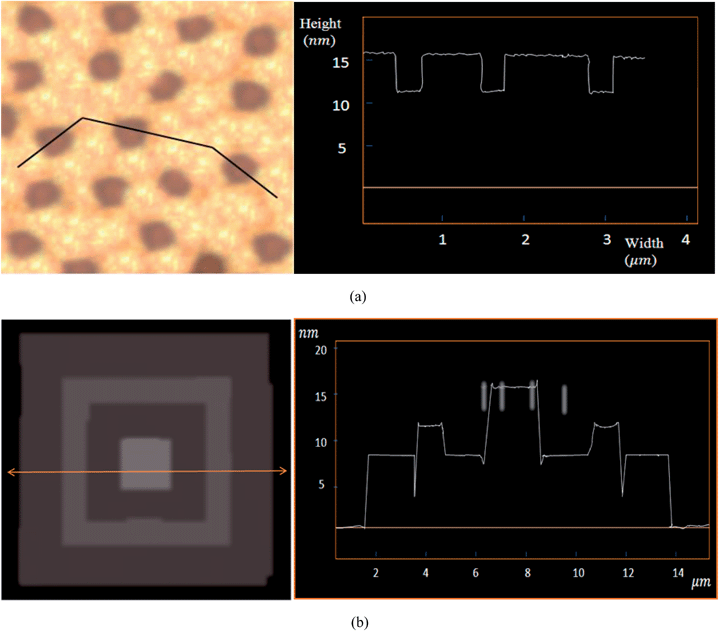
In some samples, the height variance between the matrix and dispersed phases was very low. Hence, the hardness variances could be considered as a determinant parameter in detection. Fig. 2(a) depicts the PEG5000-b-PS4600/PEG5000-b-PMMA17100 mixed-brush morphology constructed at Tc = 23 °C. The height variance between the matrix and dispersed phase regions is equal to 1.9 nm. Here, the PMMA-dispersed domains are blurred significantly in comparison with the matrix phase constructed with PS grafted chains on the substrate. Similarly, the corresponding morphology of the PEG5000-b-PS10000/PEG5000-b-PMMA13100 mixed-brush single crystal constructed at Tc = 30 °C, shown in Fig. 2(b), has a height variance of 5.7 nm. By increasing the height variance, the clarity of images increases. Fig. 2(c) is related to the surface morphology of the PEG5000-b-PS14800/PEG5000-b-PMMA17100 mixed-brush single crystal in which the height variance is equal to 6.7 nm. It appears that the last morphology has a higher resolution due to its elevated height variance between the matrix and dispersed phases.
Effect of PS block molecular weight on the thickness, tethering density and PMMA dispersed domain size
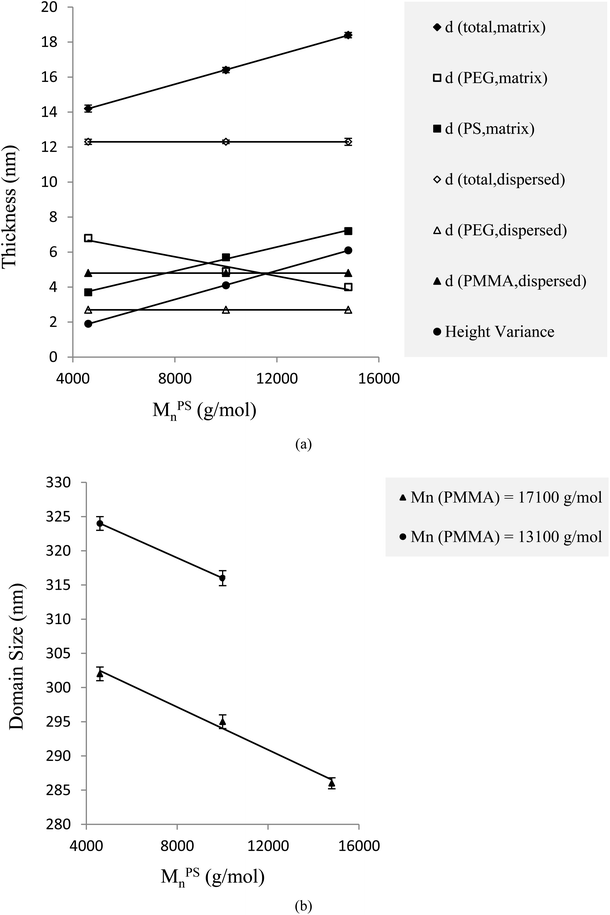
As with homo-brush single crystals, in mixed-brushes via enhancement of the amorphous block molecular weight, the total thickness due to an increase in the amorphous layer height increases because the brushes want to overcome the overlapping. However, the crystalline substrate thickness decreases.The escalation of osmotic pressure, which is exerted by a tethered chain on the surface of a single crystal substrate to enable its required surface area to be expanded, results from the need of amorphous chains to expand their coverage surface area. This reaction causes the substrate to decrease its thickness. Through this process, the folding number of crystalline chains is increased and the tethered chains on the substrate surface become distant from each other.62–66 Explanations for alterations of PS molecular weights are reported in Table S1† and Fig. 3.
By increasing MPSn while PMMA features are kept constant, the clarity of images is intensified because the height variance between PS-matrix and PMMA-dispersed phase regions increases. These processes are graphically depicted in Fig. 2(c) and 3(a). Here, the colour variance is important for identifying the various phase regions. Moreover, the features of each phase region affect only the respective thickness and tethering density. Keeping all other influencing parameters at a certain condition and changing the molecular weight of the amorphous block of PS as a type, only the overall thickness and tethering density of PS-covered areas undergo the related alterations. This relation satisfies the condition of corresponding PMMA systems as well, which is discussed in the following section. At Tc = 23 °C, the molecular weight of PS changed from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 14
000 to 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 g mol−1, whereas that of PMMA was constant at 17
800 g mol−1, whereas that of PMMA was constant at 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 g mol−1; the total thickness varied from 16.4 to 18.4 nm; and the respective tethering densities changed from 0.37 to 0.30 nm−2 (Table S1†). Therefore, the thickness (12.3 nm) and tethering density (0.20 nm−2) of the PMMA regions have remained constant. These results are graphically illustrated in Fig. 3(a) and tabulated in Table S1.† The thickness and tethering density of the PMMA domains do not change with changes in the molecular weight of the PS brushes. For example, when the molecular weight of PS was changed from 4600 to 10
100 g mol−1; the total thickness varied from 16.4 to 18.4 nm; and the respective tethering densities changed from 0.37 to 0.30 nm−2 (Table S1†). Therefore, the thickness (12.3 nm) and tethering density (0.20 nm−2) of the PMMA regions have remained constant. These results are graphically illustrated in Fig. 3(a) and tabulated in Table S1.† The thickness and tethering density of the PMMA domains do not change with changes in the molecular weight of the PS brushes. For example, when the molecular weight of PS was changed from 4600 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 while that of PMMA was fixed at 13
000 g mol−1 while that of PMMA was fixed at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 g mol−1 at Tc = 23 °C, the thickness and tethering density of PMMA domains were equal to 11.2 nm and 0.22 nm−2, respectively (Table S1†).
100 g mol−1 at Tc = 23 °C, the thickness and tethering density of PMMA domains were equal to 11.2 nm and 0.22 nm−2, respectively (Table S1†).
Domain size is defined by calculating the average of the longest and shortest lines, which connect two points on the perimeter of each domain. Regarding percentages, the somewhat insignificant tolerances from the respective average dispersed domain size are reported in all data Table S1–S3†. By increasing the PS molecular weight, the hindrance against the absorption of PEG-b-PMMA chains into the single crystal structure increases; thus, the PMMA-dispersed phase size reduces. This effect could be associated with the PS's intensified hindrance against the PMMA chains. Table S1† represents the trend of PMMA dispersed domain size changes with respect to variation of PS amorphous block molecular weights, where the molecular weights of PMMA blocks are constant, at the same growth conditions of self-seeding, crystallization temperatures and solvent quality. At Tc = 23 °C, raising the molecular weight of PS from 4600 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and finally to 14
000 and finally to 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 g mol−1 while fixing the molecular weight of PMMA at 17
800 g mol−1 while fixing the molecular weight of PMMA at 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 g mol−1, the domain size of dispersed phase decreased gradually from 302 to 295 to 286 nm. This trend is shown in Fig. 3(b). The tethering density (σ) and crystalline substrate thickness (dCRYST) can be determined from eqn (1) and (2), respectively.39,42,43
100 g mol−1, the domain size of dispersed phase decreased gradually from 302 to 295 to 286 nm. This trend is shown in Fig. 3(b). The tethering density (σ) and crystalline substrate thickness (dCRYST) can be determined from eqn (1) and (2), respectively.39,42,43
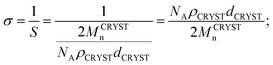 | (1) |
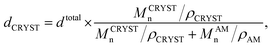 | (2) |
Influence of PMMA block molecular weight on the thickness, tethering density and the PMMA dispersed domain size
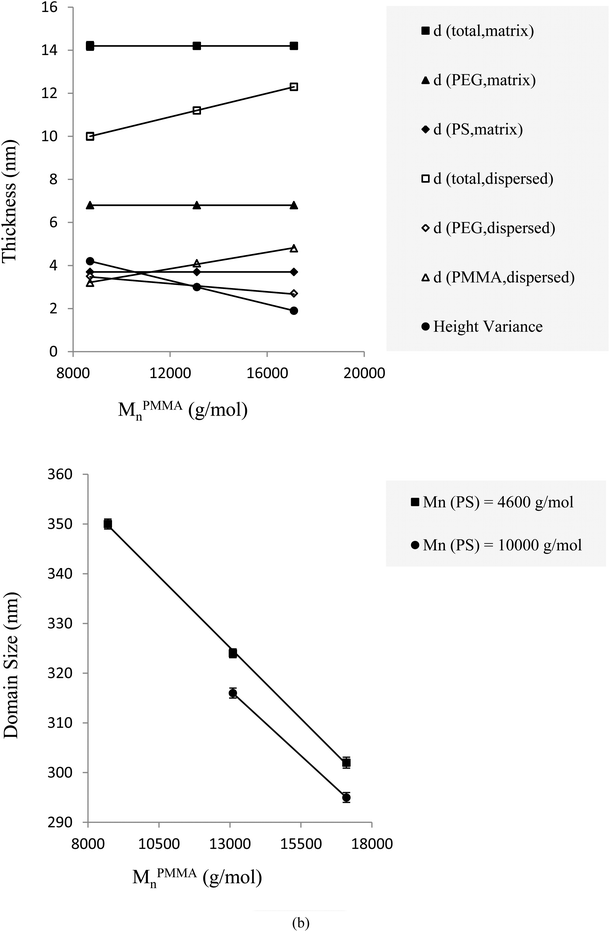
PMMA molecular weight enhancement while fixing all properties of PS tethered chains leads to deterioration of the height image clarity because the height variance decreases. This effect is shown graphically in Fig. 2(a) and 4(a). Therefore, the opaqueness and transparency of the different phase regions, which belong to PMMA-dispersed and PS-matrix phases, respectively, are more helpful. At Tc = 23 °C, when the molecular weight of PMMA was changed from 8700 to 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 g mol−1, while that of PS was kept constant at 4600 g mol−1, the total thickness varied from 10.0 to 11.2 nm. The corresponding tethering densities changed from 0.26 to 0.22 nm−2, whereas the thickness (∼14.2 nm) and tethering density (∼0.51 nm−2) of the PS regions remained constant. This trend is represented in Fig. 4(a) and in Table S2.† Moreover, although the molecular weights of PS and PMMA blocks changed somewhat similarly, alteration of the thickness and tethering density in the PS region was more pronounced than that in PMMA-covered patches because the osmotic pressure among PS chains is less than that among PMMA chains.
100 g mol−1, while that of PS was kept constant at 4600 g mol−1, the total thickness varied from 10.0 to 11.2 nm. The corresponding tethering densities changed from 0.26 to 0.22 nm−2, whereas the thickness (∼14.2 nm) and tethering density (∼0.51 nm−2) of the PS regions remained constant. This trend is represented in Fig. 4(a) and in Table S2.† Moreover, although the molecular weights of PS and PMMA blocks changed somewhat similarly, alteration of the thickness and tethering density in the PS region was more pronounced than that in PMMA-covered patches because the osmotic pressure among PS chains is less than that among PMMA chains.
With an increase in MPMMAn while all other parameters, especially the PS block molecular weight, were constant, the domain sizes of PMMA dispersed patches decreased. This trend could be attributed to the increase in hindrance of PMMA tethered chains. The increase in MPMMAn caused the tethered chains on the substrate surface to need more area to cover. This not only affects the PMMA brushes conformation but also reduces the probability of PEG-b-PMMA chain presence in the single crystal structure. Table S2† shows the trend of MPMMAn variation for constant MPSn. Increasing the molecular weight of the PMMA block from 8700 to 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 and subsequently to 17
100 and subsequently to 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 g mol−1, where MPSn was equal to 4600 g mol−1 at Tc = 23 °C, the respective domain sizes of the dispersed phase reduced from 350 to 324 to 302 nm, respectively (Fig. 4(b)).
100 g mol−1, where MPSn was equal to 4600 g mol−1 at Tc = 23 °C, the respective domain sizes of the dispersed phase reduced from 350 to 324 to 302 nm, respectively (Fig. 4(b)).
An interesting point is that the heights of the PMMA tethered chains are less than the corresponding heights of the PS brushes. This trend is satisfied even when the molecular weight of the PMMA brushes is considerably higher than that of the PS brushes (17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 g mol−1 vs. 4600 g mol−1). As described, this effect could be associated with the solvent quality and interactions with the substrate surface. In PEG5000-b-PS4600/PEG5000-b-PMMA17100 mixed-brush single crystals at Tc = 23 °C, despite having higher molecular weight, the height of the PMMA brushes was lower (12.3 vs. 14.2 nm).
100 g mol−1 vs. 4600 g mol−1). As described, this effect could be associated with the solvent quality and interactions with the substrate surface. In PEG5000-b-PS4600/PEG5000-b-PMMA17100 mixed-brush single crystals at Tc = 23 °C, despite having higher molecular weight, the height of the PMMA brushes was lower (12.3 vs. 14.2 nm).
The effect of PMMA block molecular weight on the dispersed domain size deduction was more intensified. At Tc = 23 °C, when the molecular weight of the PS block was changed from 4600 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 while that of PMMA was fixed at 13
000 g mol−1 while that of PMMA was fixed at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 g mol−1, the domain size of the dispersed phase decreased from 324 to 316 nm (Fig. 3(b)). In contrast to this smooth reducing trend, at the same Tc when MPMMAn was increased from 8700 to 13
100 g mol−1, the domain size of the dispersed phase decreased from 324 to 316 nm (Fig. 3(b)). In contrast to this smooth reducing trend, at the same Tc when MPMMAn was increased from 8700 to 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 g mol−1 while that of PS was constant (=4600 g mol−1), the domain size of PMMA dispersed phase decreased from 350 to 324 nm (Fig. 4(b)). In the latter sample, even though the hindrance of the PS chains was minimum (MPSn = 4600 g mol−1), the dispersed domain size reduction with escalating PMMA molecular weight was significant and can be attributed to the prevalent influence of the conformation of PMMA tethered chains. This comparison can be drawn from the slope of graphs of domain size vs. amorphous block molecular weight (Fig. 3(b) and 4(b)).
100 g mol−1 while that of PS was constant (=4600 g mol−1), the domain size of PMMA dispersed phase decreased from 350 to 324 nm (Fig. 4(b)). In the latter sample, even though the hindrance of the PS chains was minimum (MPSn = 4600 g mol−1), the dispersed domain size reduction with escalating PMMA molecular weight was significant and can be attributed to the prevalent influence of the conformation of PMMA tethered chains. This comparison can be drawn from the slope of graphs of domain size vs. amorphous block molecular weight (Fig. 3(b) and 4(b)).
Influence of crystallization temperature on the single crystal thickness and tethering density of mixed-brushes
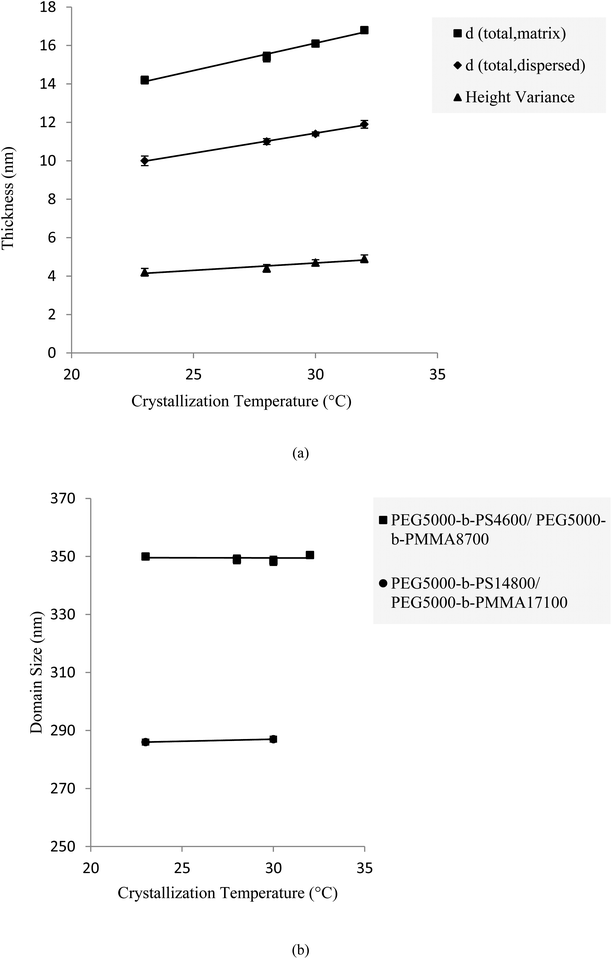
In polymer mixed-brushes as well as homo-brushes, by increasing the crystallization temperature, the total height and substrate thickness of single crystals in both matrix and dispersed phases increased. This enhancement could be attributed to the increase in thickness of the substrate and amorphous grafted brushes. Keeping the crystallization temperature (Tc) constant, the thickness of single crystal substrate (dCRYST) can be fixed because it is a function of undercooling degree (eqn (3)68–70).
 | (3) |
This equation indicates that the effective parameters for ΔTundercooling are dissolution temperature (Td) and Tc. Moreover, the thickness enhancement of the crystalline substrate could be related to the tendency of PEG chains to reach to the more stretched state (to reduce the free energy via Tc increase59). With an increase in the crystalline substrate thickness and tethered chains approaching each other, the number of tethered chains per surface area rises. The trends of thicknesses and tethering densities related to the PMMA and PS phase regions for mixed-brush surface morphologies are reported in Table S3.† By elevating the crystallization temperature, both substrate and amorphous layer heights in the PS-matrix increase more than those in the PMMA-dispersed phase. The greater increase in thickness of the crystalline substrate beneath the PS tethered chains is due to lower osmotic pressure exerted with PS chains in comparison to PMMA chains. To prove that the osmotic pressure of PS tethered chains is less than that of PMMA grafted ones, we consider two different systems. One includes PEG-b-PS diblock copolymers with the highest molecular weight of PS in amyl acetate, which is a very good solvent for PS, whereas the other includes PEG-b-PMMA diblock copolymers with the least molecular weight of PMMA in a partially poor solvent. We compared the gyration radii of PS with ![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) n = 7700 g mol−1 in a very good solvent (=3.7 nm)37 and PMMA with
n = 7700 g mol−1 in a very good solvent (=3.7 nm)37 and PMMA with ![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) n = 7800 g mol−1 in a nearly good solvent (=3.4 nm)40 with data of our growth system. In this work, the applied solvent, amyl acetate, is partially poor for PMMA, and the corresponding molecular weight of its block is significantly less than that of the PS block. Therefore, the gyration radius of PS is higher than that of PMMA. Conducting experiments with more scrutiny revealed that at the same growth conditions, the substrate thickness of the PMMA-covered single crystal is considerably less than the corresponding thickness of the PS-covered substrate. Therefore, the impact of attractive interaction between PMMA chains and the single crystal substrate is a dominant parameter affecting the substrate thickness. We speculate that the mentioned interaction could in turn have the most significant effect on the exerted osmotic pressure of chains and substrate thickness as well. As mentioned previously, alteration of the amorphous block molecular weight affects only the thickness and tethering density of that region. Therefore, we are able to make a comparison between the thickness of the PS region in PEG5000-b-PS14800/PEG5000-b-PMMA17100 and that of the PMMA region in PEG5000-b-PS4600/PEG5000-b-PMMA8700 mixed-brush single crystals at Tc = 23 °C. To exemplify the presented explanations, the thickness of the substrate beneath the PS (
n = 7800 g mol−1 in a nearly good solvent (=3.4 nm)40 with data of our growth system. In this work, the applied solvent, amyl acetate, is partially poor for PMMA, and the corresponding molecular weight of its block is significantly less than that of the PS block. Therefore, the gyration radius of PS is higher than that of PMMA. Conducting experiments with more scrutiny revealed that at the same growth conditions, the substrate thickness of the PMMA-covered single crystal is considerably less than the corresponding thickness of the PS-covered substrate. Therefore, the impact of attractive interaction between PMMA chains and the single crystal substrate is a dominant parameter affecting the substrate thickness. We speculate that the mentioned interaction could in turn have the most significant effect on the exerted osmotic pressure of chains and substrate thickness as well. As mentioned previously, alteration of the amorphous block molecular weight affects only the thickness and tethering density of that region. Therefore, we are able to make a comparison between the thickness of the PS region in PEG5000-b-PS14800/PEG5000-b-PMMA17100 and that of the PMMA region in PEG5000-b-PS4600/PEG5000-b-PMMA8700 mixed-brush single crystals at Tc = 23 °C. To exemplify the presented explanations, the thickness of the substrate beneath the PS (![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) n = 14
n = 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 g mol−1) brushes is 4.0 nm, whereas that of PMMA (
800 g mol−1) brushes is 4.0 nm, whereas that of PMMA (![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) n = 8700 g mol−1) brushes is 3.5 nm.
n = 8700 g mol−1) brushes is 3.5 nm.
Actually, two main reasons can be presented for the significant enhancement of the PS amorphous layer height. First, the increase of substrate thickness in PS growth systems is more than that of PMMA systems. Hence, the tethering density will increase significantly more in PS single crystals than in PMMA single crystals. Therefore, the steric repulsion between PS tethered chains increases significantly and consequently the chains tend to attain a more stretched conformation. In other words, due to the repulsive interaction of PS-grafted chains with the substrate surface, they are allowed to be freely stretched and move away from the surface. If we consider all previously mentioned effective parameters, we can conclude that via enhancement of crystallization temperature, the variance between PMMA and PS phase regions increases (Fig. 5(a)) and, consequently, the clarity of height images to distinguish the various phases increases. For mixed-brush morphologies created from PEG5000-b-PS10000/PEG5000-b-PMMA13100, through changing the crystallization temperature from 23 to 30 °C, the PS phase thickness changed from 16.4 to 18.2 nm, whereas the thickness of the PMMA dispersed phase increased from 11.2 to 12.5 nm (Table S3†). Another example is graphically illustrated in Fig. 5(a) for the PEG5000-b-PS4600/PEG5000-b-PMMA8700 single crystal.
Considering the data reported in Table S3,† the domain sizes of the PMMA-dispersed phase in the PS-matrix do not change via crystallization temperature alteration, which could be related to the consistency of effective parameters for the dispersed domain sizes (i.e., the molecular weight of PS and PMMA amorphous blocks).
For example, in mixed-brush morphologies created from PEG5000-b-PS14800/PEG5000-b-PMMA17100, by changing the crystallization temperature from 23 to 30 °C, the dispersed domain size was fixed to some extent at 286 nm (Fig. 5(b)). This trend satisfied the conditions for other samples as well.
Adopting the polymer epitaxial structures to verify the crystalline substrate thickness determined in mixed-brush single crystals
We grew single crystals of PEG5000-b-PS4600 and used them as the seeds for further growth of the second layer including homo-PEG5000. Subsequently, this procedure was repeated to form a nanostructure including outer layers of PEG5000-b-PMMA8700 (other PMMA blocks with other molecular weights such as 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 and 17
100 and 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 g mol−1 were applied to create the corresponding layers in the remaining samples) and homo-PEG5000. The materials of the crystalline block and homopolymer were the same; thus, epitaxial growth can occur with identical growth fronts. At the conjunction between the diblock copolymer and homopolymer single crystals, the thickness has to be confined to the same value, which is provided by previously presented growth fronts. The crystalline substrate thickness at the conjunction of the diblock copolymer and homopolymer is equal to that of diblock copolymer single crystal. However, the thermodynamic metastable thickness of these two crystals will not remain the same. As the crystal grows away from the seed, the thermodynamic thickness will be reached, which is significantly larger than the primary substrate thickness. Therefore, measurement of the thickness at the conjunction of copolymer and homopolymer crystals could provide an accurate determination for the substrate thicknesses of copolymer single crystals.39–42
100 g mol−1 were applied to create the corresponding layers in the remaining samples) and homo-PEG5000. The materials of the crystalline block and homopolymer were the same; thus, epitaxial growth can occur with identical growth fronts. At the conjunction between the diblock copolymer and homopolymer single crystals, the thickness has to be confined to the same value, which is provided by previously presented growth fronts. The crystalline substrate thickness at the conjunction of the diblock copolymer and homopolymer is equal to that of diblock copolymer single crystal. However, the thermodynamic metastable thickness of these two crystals will not remain the same. As the crystal grows away from the seed, the thermodynamic thickness will be reached, which is significantly larger than the primary substrate thickness. Therefore, measurement of the thickness at the conjunction of copolymer and homopolymer crystals could provide an accurate determination for the substrate thicknesses of copolymer single crystals.39–42
Regarding the conjunction thickness between the copolymer and homopolymer crystals, which is achievable from the height profile, we were able to verify the calculated crystalline substrate thickness in single crystals with matrix-dispersed surface morphology. When the crystals were deposited onto the surface of a hard silicon wafer for AFM analysis, the homo-PEG single crystals slipped down to the silicon surface for the gravity effect (Fig. 6(b)). Here, the thicknesses achieved at the conjunctions of the copolymer seeds are a direct measure of dPEG. The calculated thicknesses of the PMMA and the PS phases in the mixed-brush single crystals are consistent with the achieved thicknesses from respective layers of epitaxial structures. For example, considering the data of Table S3† and Fig. 6, it could be understood that in the mixed-brush single crystal of PEG5000-b-PS4600/PEG5000-b-PMMA8700, the calculated thicknesses of the PS and PMMA regions are equal to 7.7 and 4.0 nm, respectively. It is interesting to note that the corresponding achieved substrate thicknesses from the epitaxial structure for PS- and PMMA-covered areas are the same (i.e., 7.7 and 4.0 nm).
Moreover, this consistency indicates that our primary hypotheses for PS, PMMA, and PEG are completely valid. In this case, we used the bulk density of atactic PS and PMMA blocks for their respective tethered chains, and we ignored the bulk density of amorphous PEG.
Conclusions
We prepared single crystals with matrix (PS)-dispersed PMMA surface morphologies. Various conformations of PMMA and PS chains could be a reasonable cause for higher probability of the crystalline block entrance of PEG-b-PS diblock copolymer chains in crystalline structures in comparison to PEG-b-PMMA chains; in addition, these conformations could cause detectable height variances between various phase regions. By increasing the molecular weights of the PS and PMMA blocks, the dispersed domain sizes decreased due to enhancement of the hindrance against the presence of PEG-b-PMMA diblock copolymers in the single crystal structure. Lower osmotic pressure of PS tethered chains resulted in a rising trend of thickness and tethering density with increase in crystallization temperature, which was more significant in PS-covered regions. That is, after increasing the molecular weight of one of the amorphous blocks, the total and substrate thicknesses of only the related area underwent variations. Additionally, in mixed-brush single crystals of PEG5000-b-PS4600/PEG5000-b-PMMA8700, the calculated thicknesses of the PS phase and/PMMA-dispersed phase were equal to 7.7 and 4.0 nm respectively, and the corresponding achieved substrate thicknesses of the epitaxial structure for PS- and PMMA-covered areas were the same.Acknowledgements
We would like to extend our sincere gratitude to Prof. Ali Akbar Entezami and Dr Marzieh Fathi who sympathetically helped us in synthesizing the materials. Our special thanks also go to Morteza Nasiri for his invaluable discussions on kinetic study.Notes and references
- H. Kind, H. Yan, B. Messer, M. Law and P. Yang, Adv. Mater., 2002, 14, 158 CrossRef CAS.
- R. Rosario, D. Gust, M. Hayes, F. Jahnke, J. Springer and A. A. Garsia, Langmuir, 2002, 18, 8062 CrossRef CAS.
- J. Lahann, S. Mitragotri, T.-N. Tran, H. Kaido, J. Sundaram, I. S. Choi, S. Hoffer, G. A. Somorjal and R. Langer, Science, 2003, 299, 371 CrossRef CAS PubMed.
- T. P. Russel, Science, 2002, 297, 964 CrossRef PubMed.
- R. Yoshida, K. Sakai, T. Okano, Y. Sakurai, Y. H. Bae and S. W. Kim, J. Biomater. Sci., Polym. Ed., 1991, 3(2), 155 CrossRef CAS PubMed.
- V. Pan, R. Wesley, R. Lvginbuhl, D. Denton and B. Ratner, Biomacromolecules, 2001, 2, 32 CrossRef PubMed.
- Y. Nagasaki and K. Kataoka, Trends Polym. Sci., 1996, 4, 59 CAS.
- P. G. deGennes, Macromolecules, 1980, 13, 1069 CrossRef CAS.
- A. Sidorenko, S. Minko, K. Schenk-Meuser, H. Duschner and M. Stamm, Langmuir, 1999, 15, 8349 CrossRef CAS.
- D. Usov, V. Gruzdev, M. Nitschke, M. Stamm, O. Hoy, I. Luzinov, I. Tokarev and S. Minko, Macromolecules, 2007, 40, 8774 CrossRef CAS.
- F. Zhou and W. T. S. Huck, Phys. Chem. Chem. Phys., 2006, 8, 3815 RSC.
- Polymer Brushes, ed. R. C. Advincula, W. J. Brittain, K. C. Caster and J. Rühe, Wiley-VCH, Weinheim, 2004 Search PubMed.
- W. Senaratne, L. Andruzzi and C. K. Ober, Biomacromolecules, 2005, 6, 2427 CrossRef CAS PubMed.
- G. K. Jennings and E. L. Brantley, Adv. Mater., 2004, 16, 1983 CrossRef CAS PubMed.
- M. R. Tomlinson and J. Genzer, Chem. Commun., 2003, 1350 RSC.
- D. J. Dyer, Adv. Funct. Mater., 2003, 13, 667 CrossRef CAS PubMed.
- B. Zhao and W. J. Brittain, Prog. Polym. Sci., 2000, 25, 677 CrossRef CAS.
- B. P. Harris, J. K. Kutty, E. W. Fritz, C. K. Webb, K. J. L. Burg and A. T. Metters, Langmuir, 2006, 22, 4467 CrossRef CAS PubMed.
- J. Piehler, A. Brecht, K. E. Geckeler and G. Gauglitz, Biosens. Bioelectron., 1996, 11, 579 CrossRef CAS.
- S. Minko, S. Patil, V. Datsyuk, F. Simon, K.-J. Eichhorn, M. Motornov, D. Usov, I. Tokarev and M. Stamm, Langmuir, 2002, 18, 289 CrossRef CAS.
- W. K. Idol and J. L. Anderson, J. Membr. Sci., 1986, 28, 269 CrossRef CAS.
- J. U. Kim and M. W. Matsen, Eur. Phys. J. E, 2007, 23, 135 CrossRef CAS PubMed.
- T. A. Witten and P. A. Pincus, Macromolecules, 1986, 19, 2509 CrossRef CAS.
- A. M. Rouhi, Chem. Eng. News, 1999, 77, 51 Search PubMed.
- L. E. Niklason, J. Gao, W. M. Abbott, K. K. Hirschi, S. Houser, R. Marini and R. Langer, Science, 1999, 284, 489 CrossRef CAS.
- D. E. Kataoka and S. M. Trolan, Nature, 1999, 402, 794 CrossRef CAS PubMed.
- A. M. Rouhi, Chem. Eng. News, 1997, 75, 41 Search PubMed.
- F. T. Hesselink, J. Colloid Interface Sci., 1977, 60, 448 CrossRef CAS.
- S. Minko, D. Usov, M. Motornov, L. Ionov and M. Stamm, Polym. Mater. Sci. Eng., 2003, 89, 156 CAS.
- B. Zhao, Polymer, 2003, 44, 4079 CrossRef CAS.
- S. Minko, D. Usov, E. Goreshnik and M. Stamm, Macromol. Rapid Commun., 2001, 22, 206 CrossRef CAS.
- S. Minko, M. Müller, D. Usov, A. Scholl, C. Froeck and M. Stamm, Phys. Rev. Lett., 2002, 88, 035502 CrossRef CAS.
- B. Zhao and W. J. Brittain, Macromolecules, 2000, 33, 8813 CrossRef CAS.
- S. Minko, M. Müller, M. Motornov, M. Nitschke, K. Grundke and M. Stamm, J. Am. Chem. Soc., 2003, 125, 3896 CrossRef CAS PubMed.
- M. Lemieux, S. Minko, D. Usov, M. Stamm and V. V. Tsukruk, Langmuir, 2003, 19, 6126 CrossRef CAS.
- J. Wang, S. Kara, T. E. Long and T. C. Ward, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 3742 CrossRef CAS.
- Y. Chen, PhD Dissertation, University of Akron, 2005.
- H. Xiong, J. X. Zheng, R. M. Van Horn, K.-U. Jeong, R. P. Quirk, B. Lotz, E. L. Thomas, W. J. Brittain and S. Z. D. Cheng, Polymer, 2007, 48, 3732 CrossRef CAS PubMed.
- W. Y. Chen, J. X. Zheng, S. Z. D. Cheng, C. Y. Li, P. Huang, L. Zhu, H. Xiong, Q. Ge, Y. Guo, R. P. Quirk, B. Lotz, L. Deng, C. Wu and E. L. Thomas, Phys. Rev. Lett., 2004, 93, 028301 CrossRef.
- R. M. Van Horn, Ph.D. Dissertation, University of Akron, 2009.
- S. Agbolaghi, F. Abbasi and K. Jalili, J. Polym. Res., 2014 DOI:10.1007/s10965-014-0380-0.
- W. Y. Chen, C. Y. Li, J. X. Zheng, P. Huang, L. Zhu, Q. Ge, R. P. Quirk, B. Lotz, L. Deng, C. Wu, E. L. Thomas and S. Z. D. Cheng, Macromolecules, 2004, 37, 5292 CrossRef CAS.
- J. X. Zheng, H. Xiong, W. Y. Chen, K. Lee, R. M. Van Horn, R. P. Quirk, B. Lotz, E. L. Thomas, A.-C. Shi and S. Z. D. Cheng, Macromolecules, 2006, 39, 641 CrossRef CAS.
- J. Brandup and E. H. Immergut, Polymer Handbook, Wiley, New York, 1975 Search PubMed.
- B. Wunderlich, Macromolecular Physics, Academic, New York, 1973, vol. 1, Crystal Structure, Morphology, Defects Search PubMed.
- R. C. Weast, Handbook of Chemistry and Physics, CRC Press, New York, 1980 Search PubMed.
- S. Walheim, M. Boltau, J. Mlynek, G. Krausch and U. Steiner, Macromolecules, 1997, 30, 4995 CrossRef CAS.
- K. Jankova, X. Y. Chen, J. Kops and W. Batsberg, Macromolecules, 1998, 31, 538 CrossRef CAS.
- Q. Xiao, X. Zhang, J. Yi, X. Wangand and H. Zhang, Iran. Polym. J., 2008, 17, 781 CAS.
- K. Matyjaszewski and T. P. Davis, Handbook of Radical Polymerization, John Wiley and Sons, Inc., Australia, Hoboken, 2002 Search PubMed.
- B. Reining, H. Keul and H. Hocker, Polymer, 1999, 40, 3555 CrossRef CAS.
- D. J. Siegwart, W. Wu, M. Mandalaywala, M. Tamir, T. Sarbu, M. S. Silverstein, T. Kowalewski, J. O. Hollinger and K. Matyjaszewski, Polymer, 2007, 48, 7279 CrossRef CAS PubMed.
- K. Jankova, X. Y. Chen, J. Kops and W. Batsberg, Polym. Bull., 1999, 42, 153 CrossRef CAS.
- X. Sun, H. Zhang, X. Huang, X. Wang and Q.-F. Zhou, Polymer, 2005, 46, 5251 CrossRef CAS PubMed.
- B. Lotz and A. J. Kovacs, Kolloid Z. Z. Polym., 1966, 209(2), 97 CAS.
- B. Lotz, A. J. Kovacs, G. A. Bassett and A. Keller, Kolloid Z. Z. Polym., 1966, 209, 115 CAS.
- F. J. BaltáCalleja, I. L. Hay and A. Keller, J. Polym. Sci., 1964, A2, 2171 Search PubMed.
- F. J. BaltáCalleja, I. L. Hay and A. Keller, Kolloid Z. Z. Polym., 1966, 209, 128 Search PubMed.
- J. Chen, S. Z. D. Cheng, S. S. Wu, B. Lotz and J.-C. Wittmann, J. Polym. Sci., Polym. Phys. Ed., 1995, 33, 1851 CrossRef CAS PubMed.
- H. Hamie, Ph.D. Dissertation, University of Haute Alsace, 2010.
- P. H. Richardson, R. W. Richard, D. J. Blundell, W. A. MacDonald and P. Mills, Polymer, 1995, 36, 3059 CrossRef CAS.
- H. J. Taunton, C. Toprakcioglu, L. J. Fetters and J. Klein, Nature, 1988, 332, 712 CrossRef CAS.
- S. Granick and J. Herz, Macromolecules, 1985, 18, 460 CrossRef CAS.
- M. S. Kent, L. T. Lee, B. Farnoux and F. Rondelez, Macromolecules, 1992, 25, 6240 CrossRef CAS.
- B. Bhushan, J. N. Israelachvili and U. Landman, Nature, 1995, 374, 607 CrossRef CAS.
- S. T. Milner, Science, 1991, 251, 905 CrossRef CAS PubMed.
- J. Maul, B. G. Frushour, J. R. Kontoff, H. Eichenauer, K.-H. Ott and C. Schade, Polystyrene and Styrene Copolymers, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2007 Search PubMed.
- J. I. Lauritzen and J. D. Hoffman, J. Res. Natl. Bur. Stand., Sect. A, 1960, 64, 73 CrossRef.
- J. D. Hoffman and J. I. Lauritzen, J. Res. Natl. Bur. Stand., Sect. A, 1961, 65A, 297 CrossRef CAS.
- B. Wunderlich, Macromolecular Physics, Academic Press, New York, 1980 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra00086b |
| This journal is © The Royal Society of Chemistry 2014 |