The ternary amide KLi3(NH2)4: an important intermediate in the potassium compound-added Li–N–H systems†
Bao-Xia Dong,
Liang Song,
Jun Ge,
Yun-Lei Teng* and
Shi-Yang Zhang
College of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou, 225002, P. R. China. E-mail: ylteng@yzu.edu.cn
First published on 4th February 2014
Abstract
In this paper, the KH-added LiH–NH3, KH-added LiH–LiNH2, KH-added LiNH2, and KNH2-added LiNH2 systems were systematically investigated. It was found that the ternary amide KLi3(NH2)4 was an important intermediate that was inclined to be formed in the dehydrogenation and hydrogenation processes of the potassium compound-added Li–N–H system. Further investigations revealed that both the solid state reaction of LiNH2 with KNH2 and the solid state reaction of LiNH2 with KH under mechanical ball milling or heat treatment conditions will lead to the formation of the KLi3(NH2)4 ternary amide. Moreover, the ternary amide KLi3(NH2)4 single phase was successfully synthesized by the mechanical ball milling and its ammonia desorption and hydrogenation properties were investigated. It was observed that the ammonia desorption rate of KLi3(NH2)4 was faster than that of LiNH2 and the hydrogen absorption kinetics of KLi3(NH2)4 were between those of KNH2 and LiNH2.
1. Introduction
The safe and efficient storage of hydrogen represents one of the most significant technological challenges to the widespread adoption of a hydrogen-based energy economy. Chemical hydrogen storage materials, due to their high hydrogen contents, are expected to be potential hydrogen sources for fuel cells. Among them, nitrogen- and boron-based compounds, such as LiNH2–LiH, N2H4, and NH3BH3 have attracted much attention.1–3The metal–N–H hydrogen storage system has been investigated over the world since it was first reported by Chen et al. in 2002, who indicated that Li3N reversibly stores over 10 mass% hydrogen in the two consecutive reactions (reaction (1)).4
| Li3N + 2H2 ↔ Li2NH + LiH + H2 ↔ LiNH2 + 2LiH | (1) |
Later, the lithium amide (LiNH2)–lithium hydride (LiH) system is also proposed as a sound solid-state storage system, as it offers relatively high gravimetric storage capacity (approximately 6.5 weight percent hydrogen released from reaction (2)).5–7
| Li2NH + H2 ↔ LiNH2+ 2LiH | (2) |
Whilst this system exhibits a relatively advantageous set of target thermodynamic parameters, the temperatures required for the dehydrogenation of lithium amide and the hydrogenation of lithium imide are still too high for the application of this system as a commercial hydrogen store. Various efforts have been devoted to improve its hydrogen absorption and desorption kinetics.8–14 It has been demonstrated recently that potassium compounds, including potassium hydride and potassium amide, possess superior catalytic effects on the improvement of hydrogenation/dehydrogenation kinetics of metal–N–H system.8,9 The hydrogen desorption/absorption kinetics for the LiH–NH3 system can be improved drastically by addition of 5 mol% KH.8 In addition, remarkable enhancement in the kinetics of dehydrogenation was achieved by introducing 3 mol% KH into the Mg(NH2)2–2LiH system, of which a ternary amide phase KLix(NH2)y may play important roles in the dehydrogenation process.9 Such an improvement was further confirmed recently by introducing KH into the 2LiNH2–MgH2 system.10 Unfortunately, the improving mechanisms of hydrogen storage performance for the potassium-added metal–N–H system have not been clarified completely so far. The investigation on potassium intermediate compounds would provide valuable information for understanding the reaction mechanisms of potassium-catalyzed metal–N–H hydrogen storage system.
In present work, the KH-added LiH–NH3, KH-added LiH–LiNH2, KH-added LiNH2, and KNH2-added LiNH2 systems were systematically investigated. It was found that the ternary amide KLi3(NH2)4 was an important intermediate that was inclined to be formed in the KH or KNH2-added Li–N–H system. The possible formation mechanism was clarified on the basis of experimental results. Moreover, the ternary amide KLi3(NH2)4 single phase was successfully synthesized by the mechanical ball milling and its ammonia desorption and hydrogenation properties were investigated.
2. Experimental procedure
2.1. Sample preparation
Lithium hydride (LiH) (98%, J&K Chemical Ltd., China), lithium amide (LiNH2) (95%, Aldrich), and NH3 (99.999%) were used for the following experiments. As additives, potassium hydride (KH) (99.5%, Aldrich) and potassium amide (KNH2) (synthesized from the KH and NH3) were chosen. The additives were dispersed into the samples by the following mechanical ball-milling method. A weighed amount of LiH or LiNH2, together with 30 steel balls (6 mm in diameter) and each additive, was put into a milling vessel made of steel of which the inner volume is about 50 cm3, where the amount of additive was 5 mol% to 300 mg of LiH or LiNH2. And then, the ball milling was performed under 0.1 MPa argon (>99.999%) atmosphere for 2 hours using a planetary ball mill apparatus (QM-3SP4). The ball-to-powder weight ratio was about 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. To minimize the temperature increment of the samples, the milling process was paused for 30 minutes every hour of milling. The hand-milled samples were obtained with a pestle in an agate mortar and the powdering time was about 30 minutes. All the samples were handled in a Ar-filled (>99.999%) glove box (Mikrouna, China) equipped with a circulative purification system, in which the typical H2O/O2 levels are below 0.1 ppm.
1. To minimize the temperature increment of the samples, the milling process was paused for 30 minutes every hour of milling. The hand-milled samples were obtained with a pestle in an agate mortar and the powdering time was about 30 minutes. All the samples were handled in a Ar-filled (>99.999%) glove box (Mikrouna, China) equipped with a circulative purification system, in which the typical H2O/O2 levels are below 0.1 ppm.
2.2. Experimental techniques
The reactions of KH-added LiH with ammonia were performed as follows. A weighed amount of 5 mol% KH-added LiH was packed into a pressure vessel. NH3 pressure of 0.5 MPa with a ratio of NH3/MH (M = Li and K) = 1 mol mol−1 was introduced into the vessel at 100 °C. Ammonia (m/z = 16) release of the just synthesized KLi3(NH2)4 and raw LiNH2 was monitored using mass spectroscopy (MS; Netzsch QMS 403 D Aëolos®, Germany) attached to a synchronous thermal analysis (DSC/DTA-TG; Netzsch STA 449 F3 Jupiter®). In the analysis, high purity argon (>99.999%) was flowed as a carrier gas, and the heating rate was fixed at 10 °C min−1. The H2 absorption conditions of MNH2 (M = Li or K) were examined as follows. A weighed alkali amide (KNH2, LiNH2 or KLi3(NH2)4) was treated at the designated temperature for 4 hours under 0.5 MPa of H2 flow condition (open system) to examine the reactivity. The sample masses before and after the experiments were measured to calculate the reaction yield.The structural characters of the produced composites were examined by X-ray diffraction (XRD) measurement (AXS D8 ADVANCE, Bruker, German) in Testing Center of Yangzhou University. The samples were covered by a polyimide sheet to protect the samples from an oxidation during measurements. The N–H stretching modes of the amides were characterized by Fourier Transform IR spectrometer (FTIR) (TENSOR 27, Bruker, Canada) in transmission mode. The test samples were prepared by cold pressing a mixture of power samples and potassium bromide (KBr) powder at a weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 to form a pellet. Each spectrum was created from 16 scans with a scan resolution of 4 cm−1. The morphologies of the samples were characterized by a scanning electron microscope (S-4800II, Hitachi, Japan). X-ray photoelectron spectroscopy (XPS) analysis was performed on a PHI 5000 VersaProbe system using monochromatic Al Ka radiation (1486.6 eV) at an accelerating power of 15 kW.
20 to form a pellet. Each spectrum was created from 16 scans with a scan resolution of 4 cm−1. The morphologies of the samples were characterized by a scanning electron microscope (S-4800II, Hitachi, Japan). X-ray photoelectron spectroscopy (XPS) analysis was performed on a PHI 5000 VersaProbe system using monochromatic Al Ka radiation (1486.6 eV) at an accelerating power of 15 kW.
3. Results and discussion
3.1. The assignment of the KLi3(NH2)4 ternary amide in the Li–N–H system
Ammonia (NH3) is one of the attractive hydrogen storage and transportation materials because it has a high hydrogen storage capacity of 17.8 mass% and is easily liquefied by compression under 1.0 MPa pressure at room temperature.15,16 Reaction (3) was shown to be ultrafast and exothermic, leading to full consumption of NH3 by LiH, and therefore this system has been recognized as a good Li–N–H hydrogen storage system.| LiH + NH3 → LiNH2 + H2 | (3) |
It was found that, when a little amount of KH (5 mol%) was added into the LiH–NH3 hydrogen storage system, the hydrogen desorption kinetics of this system at 100 °C was drastically improved by the “pseudo-catalytic” effect of KH.8 Studies on potassium intermediate compounds formed in the KH-added LiH–NH3 system will contribute to understanding the improving mechanism of hydrogen desorption kinetics. Therefore, the following two experiments were designed and performed. After the reaction between the KH-added LiH and NH3 at 100 °C for 60 minutes: (1) the gas inside the vessel was evacuated immediately; (2) gas evacuation was carried out after decreasing the temperature to room temperature and keeping for 15 minutes. The products obtained by two different operations were identified by XRD. As shown in Fig. 1, for the first experiment, the diffraction peaks of LiNH2, LiH and KH were observed, but there were no diffraction peaks of KNH2. A weak diffraction peak located at 33.4° was found, which was assigned to KLi3(NH2)4. In contrast, new diffraction peaks at 12.8, 16.5, 33.4, 58.9 and 62.1° due to KLi3(NH2)4 become obvious in the second experiment, which demonstrates that the ternary amide KLi3(NH2)4 is formed in the KH-added LiH–NH3 system during dehydrogenation. The interactions of LiH, LiNH2, KH, and KNH2, which is involved in the dehydrogenation process of KH-added LiH–NH3 system may result in the formation of KLi3(NH2)4 phase. This phenomenon implies that the KLi3(NH2)4 ternary amide would be formed in the metal–N–H hydrogen storage system including KH, LiH and NH3 and may play important roles in reaction process.
Since remarkable enhancement in the kinetics of dehydrogenation can be achieved by introducing 3 mol% KH into the Mg(NH2)2–2LiH system, hydrogen storage performances of the LiNH2–LiH system may be improved by doping a little amount of KH. The interactions of KH with LiH–LiNH2 system was also studied here. Structure examinations have been performed on the 3 mol% and 5 mol% KH-added LiH–LiNH2 systems by means of XRD. It is noted that the diffraction peaks of KH almost can not be observed for the KH-added samples after ball milling (Fig. 2a and b). However, a new diffraction peak at 33.4° due to KLi3(NH2)4 appeared. As shown in Fig. 2, for the 5 mol% KH-added LiH system after ball milling, the diffraction peaks of KH keep obvious and sharp in the XRD patterns of Fig. 2c, indicating that KH is difficult to become the amorphous form by ball milling for 2 hours. The diffraction peaks located at 33.5 and 56.1° in Fig. 2c are not due to KLi3(NH2)4 phase, but due to Li2O phase that is the contamination produced during ball milling. The results demonstrate that the KLi3(NH2)4 ternary amide will be formed in the KH-added LiNH2–LiH system after ball milling and the interaction of KH with LiNH2 may result in the formation of this ternary amide.
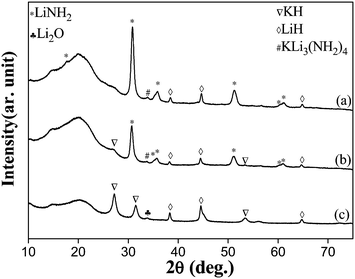 | ||
| Fig. 2 XRD patterns of LiNH2 and LiH systems with 3 mol% KH added (a) or 5 mol% KH added (b) and the 5 mol% KH-added LiH system (c) after ball milling. | ||
3.2. The possible formation mechanism of the KLi3(NH2)4 phase
With the aim of understanding the formation mechanism of KLi3(NH2)4 phase in both KH-added LiH–NH3 system and KH-added LiH–LiNH2 system, the ball milled 5 mol% KH-added LiNH2 and 5 mol% KNH2-added LiNH2 were characterized by XRD and FTIR. As shown in Fig. 3a and c, the diffraction peaks of KH and KNH2 can not be observed after ball milling, but a new weak peak located at 33.4° due to KLi3(NH2)4 phase appears for the both cases. To confirm the existence of KLi3(NH2)4 phase in the KH-added or KNH2-added LiNH2 sample after ball milling, two additional XRD experiments were conducted, as shown in Fig. 3. The KH-added LiNH2 and KNH2-added LiNH2 samples after ball milling were subjected to recrystallization at 200 °C under Ar atmosphere. After recrystallization, the diffraction peaks of KLi3(NH2)4 phase, where are mainly located at 35.0, 33.4, 16.5 and 12.8°, become obvious and sharp (Fig. 3b and d), which confirmed the existence of the KLi3(NH2)4 phase in both the samples of KH-added LiNH2 and KNH2-added LiNH2 after ball milling. For the amide and imide compounds, the N–H stretching modes are active for the FTIR spectrum. As shown in Fig. S1,† for the 5 mol% KH-added LiNH2 after ball milling, only the N–H stretching vibration frequencies of LiNH2 at 3312/3258 cm−1 are observed. Peaks due to the N–H stretching vibration frequency of KLi3(NH2)4 may be overlapped by that of LiNH2 (Fig. S1a†). However, after treatment under Ar atmosphere at 200 °C for 4 hours, a new N–H stretching vibration frequency at 3298 cm−1 due to KLi3(NH2)4 brings about (Fig. S1b†), which indicates that KH reacts with LiNH2 to produce KLi3(NH2)4 ternary amide. In the case of the 5 mol% KNH2-added LiNH2 after ball milling, the new peak at 3298 cm−1 due to the N–H stretching vibration frequency of the KLi3(NH2)4 ternary amide can be observed besides those of LiNH2 at 3312/3258 cm−1 (Fig. S1c†), indicating that KNH2 reacts with LiNH2 forming the KLi3(NH2)4 under the ball milling condition. After treatment under Ar atmosphere at 200 °C for 4 hours, the N–H stretching vibration frequency at 3298 cm−1 becomes more obvious and sharper (Fig. S1d†). The above discussion indicates that both KH and KNH2 can interact with LiNH2 to form KLi3(NH2)4 under mechanical ball milling condition according to the reactions (4) and (5), respectively.| KH + 4LiNH2 → KLi3(NH2)4 + LiH | (4) |
| KNH2 + 3LiNH2 → KLi3(NH2)4 | (5) |
In addition, the interaction of KH or KNH2 with LiNH2 under heat-treatment condition was also investigated. Firstly, the sample of KH/LiNH2 with molar ratio of 1/4 and the sample of KNH2/LiNH2 with molar ratio of 1/3 were hand-milled for 30 minutes in the glove box. Then, the two hand-milled samples were treated at 200 °C under 0.1 MPa Ar atmosphere for 48 hours. As shown in Fig. 4a and c, except the diffraction peaks of reactants, no new diffraction peaks appeared in the corresponding XRD patterns, indicating that both KH and KNH2 do not react with LiNH2 under hand-milled condition. However, obvious diffraction peaks of KLi3(NH2)4 were observed for the two samples after heat-treatment (Fig. 4b and d), implying that both KH and KNH2 can interact with LiNH2 to form the KLi3(NH2)4 under heat-treatment condition. To further prove the presence of proposed intermediate (the ternary amide KLi3(NH2)4), the products after hand milled and heat-treatment were characterized by FTIR. As shown in Fig. S2,† no new absorptions appeared for the two hand milled samples (Fig. S2a and c†), but new absorption at 3298 cm−1 due to KLi3(NH2)4 was clearly observed for the samples after heat-treatment (Fig. S2b and d†), which consists with the characterization using XRD.
In this work, XPS was also applied to further prove the presence of the proposed intermediate (the ternary amide KLi3(NH2)4). The Li 1s spectra for the LiNH2, the just synthesized KLi3(NH2)4 single phase and the potassium compound-added LiNH2 systems were shown in Fig. S3.† A XPS peak at 54.37 eV due to Li 1s of the LiNH2 is observed, as shown in Fig. S3(a).† However, the position of the Li 1s peak for the just synthesized KLi3(NH2)4 single phase is about 53.88 eV, which slightly shifts to lower binding energies (Fig. S3(b)†). It is known that the XPS shapes and positions associated with a particular metal ion depend on its valence state and electron densities between metal ions and donor atoms around. The difference of Li 1s spectra between LiNH2 and KLi3(NH2)4 may result from the different nature of the lithium–amino bond as well as different position of lithium ions that it occupies in the lattice.23,24 For the products of KH/LiNH2 with molar ratio of 1/4 after heat-treatment at 200 °C, the 1s binding energy of Li in the samples split into two peaks as 54.01 eV and 53.26 eV, which correspond to the Li 1s peaks of LiNH2 and KLi3(NH2)4, respectively (Fig. S3(c)†). For the products of KNH2/LiNH2 with molar ratio of 1/3 after heat-treatment at 200 °C, the 1s binding energy of Li in the samples split into two peaks as 54.25 eV and 53.26 eV, which corresponded to the Li 1s peaks of LiNH2 and KLi3(NH2)4, respectively (Fig. S3(d)†).
Therefore, we concluded that both KNH2 and KH can interact with LiNH2 to form KLi3(NH2)4 under mechanical ball milling or heat-treatment condition. These phenomena suggest that KLi3(NH2)4 is a relatively stable ternary amide compound that is inclined to be formed in the hydrogen storage system including KH (or KNH2) and LiNH2. Recently, Liu et al. have also found the KLi3(NH2)4 and proved that KLi3(NH2)4 played important roles in the process of hydrogen storage for the potassium compounds-catalyzed metal–N–H systems.22 They proposed that the interactions of LiNH2, Li2Mg2N3H3, and KH will result in the formation of KLi3 (NH2)4. In our opinion, the formation of KLi3(NH2)4 under that condition may result from the interaction of LiNH2 with KH or KNH2.
Similarly, a few kinds of ternary amide or imide phases such as Li2Mg(NH2)2, Li2Ca(NH2)2, K2Mg(NH2)4 and KMg(NH)(NH2) were found as intermediates playing very important roles in the hydrogen desorption of metal–N–H hydrogen storage system.17–21 KLi3(NH2)4 represents the first ternary amide phase found in the potassium compound-added Li–N–H system, which may play important roles in the process of hydrogen storage.
3.3. The properties of the KLi3(NH2)4 ternary amide
Since KLi3(NH2)4 is inclined to be formed in the potassium compounds-added Li–N–H system, it is necessary to investigate its properties for elucidating its possible roles in the process of hydrogen storage. The KLi3(NH2)4 ternary amide single crystal was prepared by the reaction of ammonia with the metals in high-pressure autoclaves as early as 1984.23 The ternary amide phase (KLi3(NH2)4) was observed after ball milling the KNH2-added LiNH2, which indicates that the KLi3(NH2)4 single phase can be produced using the mixture of KNH2 and LiNH2 with 1/3 molar ratio under the mechanochemical conditions, as expressed by reaction (4). The powder XRD profile for the synthesized KLi3(NH2)4 phase by this new method was given in Fig. 5. Apparently, the profile obtained shows the single phase. In addition, it was reported recently that a similar ternary amide phase of NaLi3(NH2)4 can also be prepared in the same way.24,25SEM was performed to examine the morphologies and particle size of the samples. For the raw LiNH2 and KNH2, the particles are not very regular in shape and size. Especially for the KNH2, the surface of KNH2 is very coarser compared with LiNH2, as shown in Fig. S4(a and b).† The particle size of the samples is about 1–10 um in average. However, after the LiNH2 and KNH2 reacted with each other in molar ratio of 1/3 by ball milling for 8 h, the particles of the product (the KLi3(NH2)4 single phase) become nearly-oval flakes and the size of most particles is more uniform and less than 500 nm (Fig. S4(c)).†
The just synthesized KLi3(NH2)4 single phase was also characterized by FTIR. As shown in Fig. 6, the N–H stretching vibration frequency of KLi3(NH2)4 at 3298 and 3253 cm−1 is about 14 and 5 cm−1 red-shifted from that of LiNH2, respectively, which indicates that, compared with those of LiNH2, the N–H bonds in KLi3(NH2)4 are weakened. The weakened N–H bonds may facilitate further interaction of KLi3(NH2)4 with LiH, which may result in the enhancement of dehydrogenation kinetics of the metal–N–H system. However, the N–H stretching vibration frequency of KLi3(NH2)4 is about 40 and 46 cm−1 blue-shifted from those of KNH2, respectively (Fig. 6), suggesting that N–H bonds of KLi3(NH2)4 are much more stable than those of KNH2.
Temperature-programmed desorption (TPD) of the KLi3(NH2)4 single phase was performed to investigate its thermal decomposition properties. As shown in Fig. 7, decomposition curve of LiNH2 was also involved for comparison. Clearly, the ammonia desorption behavior of KLi3(NH2)4 is different from that of LiNH2. For KLi3(NH2)4, ammonia desorption shows three distinct peaks. The initial ammonia desorption peak around 92 °C with an onset temperature of approximate 65 °C is a broad peak, followed by a small peak at approximate 167 °C and a large broader peak at approximate 260 °C. It is noteworthy that the onset ammonia desorption temperature and all the peaks of ammonia desorption for KLi3(NH2)4 is much lower than that of LiNH2, indicating that the ammonia desorption rate of KLi3(NH2)4 is faster than that of LiNH2. It can be inferred that, if KLi3(NH2)4 and LiNH2 coexist under heat-treatment condition, KLi3(NH2)4 is much easier to release ammonia.
 | ||
| Fig. 7 Temperature dependence of NH3 (m/z 16) desorption from LiNH2 (a) and the KLi3(NH2)4 single phase (b), the temperature was increased at a rate of 5 °C min−1. | ||
The hydrogen absorption property of KLi3(NH2)4 was also investigated and compared with those of KNH2 and LiNH2. All the amide samples were treated under H2 flow condition at the designated temperature for 4 hours. The reaction yield on the hydrogen absorption reaction of ball milled KNH2, KLi3(NH2)4, and LiNH2 for 4 hours at 100, 200, and 300 °C was shown in Fig. 8. The post-milled KNH2 and LiNH2 show the fastest and slowest reactivity, respectively, which is largely faster than that of the corresponding raw sample reported in the previous articles.26 The KLi3(NH2)4 shows a little faster reactivity at 100 °C than that of LiNH2 because of the doping of KNH2 with superior reactivity. The reaction yield at 200 °C is 97.2%, 36.7%, and 24.3% for KNH2, KLi3(NH2)4, and LiNH2, respectively. Due to the increase of the heat-treatment temperature, the increases of reaction yield, compared with that at 100 °C, are 11.4%, 31.5%, and 23.1% for KNH2, KLi3(NH2)4, and LiNH2, respectively. It is noteworthy that the hydrogen absorption kinetics is better in order of KNH2 > KLi3(NH2)4 > LiNH2 and KLi3(NH2)4 shows much faster reactivity than that of post-milled LiNH2 at 200 °C, which indicates that, if KLi3(NH2)4 and LiNH2 coexist in H2 atmosphere, KLi3(NH2)4 is preferential to react with H2.
 | ||
| Fig. 8 Hydrogenation profile for MNH2 (the KNH2, KLi3(NH2)4 and LiNH2) under H2 flow at 100, 200, and 300 °C for 4 hours. | ||
4. Conclusions
In summary, the KH-added LiH–NH3, KH-added LiH–LiNH2, KH-added LiNH2, and KNH2-added LiNH2 systems were systematically investigated. It was found that the ternary amide KLi3(NH2)4 was an important intermediate that was inclined to be formed in dehydrogenation and hydrogenation processes of the potassium compounds-added Li–N–H system. Further investigations revealed that both the solid state reaction of LiNH2 with KNH2 and the solid state reaction of LiNH2 with KH under mechanical ball milling or heat treatment condition will lead to the formation of the KLi3(NH2)4 ternary amide. Moreover, the ternary amide KLi3(NH2)4 single phase was successfully synthesized by the mechanical ball milling and its ammonia desorption and hydrogenation properties were investigated. TPD experiments shows that the ammonia desorption rate of KLi3(NH2)4 is faster than that of LiNH2. Furthermore, it was observed that the hydrogen absorption kinetics is better in order of KNH2 > KLi3(NH2)4 > LiNH2.Acknowledgements
The authors thank the reviewers for their valuable comments and suggestions. This work was financially supported by the NNSF of China (no. 21301152, 21371150), Foundation from the Priority Academic Program Development of Jiangsu Higher Education Institutions, Fostering Foundation of Yangzhou University (no. 2012CXJ012, 2012CXJ017).Notes and references
- L. Schlapbach and A. Züttel, Nature, 2001, 414, 353 CrossRef CAS PubMed.
- S. K. Singh and Q. Xu, J. Am. Chem. Soc., 2009, 131, 18032 CrossRef CAS PubMed.
- A. Aijaz, A. Karkamkar, Y. J. Choi, N. Tsumori, E. Rönnebro, T. Autrey, H. Shioyama and Q. Xu, J. Am. Chem. Soc., 2012, 134, 13926 CrossRef CAS PubMed.
- P. Chen, Z. Xiong, J. Luo, J. Lin and K. L. Tan, Nature, 2002, 420, 302 CrossRef CAS PubMed.
- P. Chen, Z. Xiong, J. Luo, J. Lin and K. L. Tan, J. Phys. Chem. B, 2003, 107, 10967 CrossRef CAS.
- S. I. Orimo, Y. Nkamori, J. R. Eliseo, A. Züttel and C. M. Jensen, Chem. Rev., 2007, 107, 4111 CrossRef CAS PubMed.
- S. Nayebossadri, K. F. Aguey-Zinsou and Z. Guo, Int. J. Hydrogen Energy, 2011, 36, 7920 CrossRef CAS PubMed.
- Y. Teng, T. Ichikawa, H. Miyaoka and Y. Kojima, Chem. Commun., 2011, 47, 12227 RSC.
- J. Wang, T. Liu, G. Wu, W. Li, Y. Liu, C. M. Araújo, R. H. Scheicher, A. Blomqvist, R. Ahuja, Z. Xiong, P. Yang, M. Gao, H. Pan and P. Chen, Angew. Chem., Int. Ed., 2009, 48, 5828 CrossRef CAS PubMed.
- W. Luo, V. Stavila and L. E. Klebanoff, Int. J. Hydrogen Energy, 2012, 37, 6646 CrossRef CAS PubMed.
- W. I. F. David, M. O. Jones, D. H. Gregory, C. M. Jewell, S. R. Johnson, A. Wallton and P. P. Edwards, J. Am. Chem. Soc., 2007, 129, 1594 CrossRef CAS PubMed.
- Y. Liu, K. Zhong, K. Luo, M. Gao, H. Pan and Q. Wang, J. Am. Chem. Soc., 2009, 131, 1862 CrossRef CAS PubMed.
- J. Wang, H. Li, S. Wang, X. Liu, Y. Li and L. Jiang, Int. J. Hydrogen Energy, 2009, 34, 1411 CrossRef CAS PubMed.
- S. Nayebossadri, Int. J. Hydrogen Energy, 2011, 36, 8335 CrossRef CAS PubMed.
- Y. Hu and E. Ruckenstein, J. Phys. Chem. A, 2003, 107, 9737 CrossRef CAS.
- T. Ichikawa, N. Hanada, S. Isobe, H. Leng and H. Fujii, J. Phys. Chem. B, 2004, 108, 7887 CrossRef CAS.
- Z. Xiong, G. Wu, J. Hu and P. Chen, Adv. Mater., 2004, 16, 1522 CrossRef CAS.
- J. Wang, G. Wu, Y. S. Chua, J. Guo, Z. Xiong, Y. Zhang, M. Gao, H. Pan and P. Chen, ChemSusChem, 2011, 4, 1622 CrossRef CAS PubMed.
- H. Wu, J. Am. Chem. Soc., 2008, 130, 6515 CrossRef CAS PubMed.
- G. Wu, Z. Xiong, T. Liu, Y. Liu, J. Hu and P. Chen, Inorg. Chem., 2007, 46, 517 CrossRef PubMed.
- J. Hu, M. Fichtner and P. Chen, Chem. Mater., 2008, 20, 7089 CrossRef CAS.
- Y. Liu, L. Chao, B. Li, M. Gao and H. Pan, J. Phys. Chem. C, 2013, 117, 866 CAS.
- H. Jacobs and B. Harbrecht, Z. Anorg. Allg. Chem., 1984, 518, 87 CrossRef CAS.
- R. L. Lowton, M. O. Jones, W. I. F. David, S. R. Johnson, M. Sommariva and P. P. Edwards, J. Mater. Chem., 2008, 18, 2355 RSC.
- H. Jacobs and B. J. Harbrecht, J. Less-Common Met., 1982, 85, 87 CrossRef CAS.
- H. Yamamoto, H. Miyaoka, S. Hino, H. Nakanishi, T. Ichikawa and Y. Kojima, Int. J. Hydrogen Energy, 2009, 34, 9760 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra00077c |
| This journal is © The Royal Society of Chemistry 2014 |





