A water-soluble highly sensitive and selective fluorescent sensor for Hg2+ based on 2-(2-(8-hydroxyquinolin)-yl)benzimidazole via ligand-to-metal charge transfer (LMCT)†
Keli Zhonga,
Xue Zhoua,
Ruibin Houb,
Pei Zhoua,
Shuhua Houa,
Yanjiang Biana,
Gang Zhangc,
Lijun Tang*a and
Xiaohong Shang*c
aDepartment of Chemistry, Liaoning Provincial Key Laboratory for the Synthesis and Application of Functional Compounds, Bohai University, Jinzhou 121013, China. E-mail: ljtang@bhu.edu.cn; Fax: +86-416-3400158; Tel: +86-416-3400302
bSchool of Chemistry and Life Sciences, Changchun University of Technology, Changchun 130012, China
cState Key Laboratory of Theoretical and Computational Chemistry, Institute of Theoretical Chemistry, Jilin University, Changchun 130023, China. E-mail: shangxiaohong58@aliyun.com
First published on 12th March 2014
Abstract
A water-soluble fluorescent sensor (L) based on 2-(2-(8-hydroxyquinolin)-yl)benzimidazole has been synthesized and characterized. Sensor L displays highly selective and sensitive recognition of Hg2+ with a fluorescence “ON–OFF” response in buffered aqueous solution (1% dimethyl sulfoxide (DMSO), Tris–HCl 10 mM, pH = 7.4). X-ray crystal structure of the L–Hg2+ complex reveals that the oxygen and nitrogen atoms of 8-hydroxyquinoline and the imine N atom of the benzimidazole unit (N1) bind Hg2+ through a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry. The fluorescence quenching mechanism is qualitatively evaluated by quantum chemical calculations which show that the fluorescence quenching phenomenon is caused by ligand-to-metal charge transfer (LMCT) in the excited state.
1 binding stoichiometry. The fluorescence quenching mechanism is qualitatively evaluated by quantum chemical calculations which show that the fluorescence quenching phenomenon is caused by ligand-to-metal charge transfer (LMCT) in the excited state.
Introduction
Hg2+ is a chemical with biological toxicity.1 The harmful effects of Hg2+ on the human body are primarily concentrated in the digestive system, central nervous system, and internal organs due to its high affinity to the thiol group in proteins,2 and it has some influence on the eyes, skin, blood and respiratory system.3 It has become one of the most notable environmental pollutants in the world due to its persistence, easy mobility, and high bioaccumulation.4 Therefore, accurate detection of trace levels of Hg2+ in water near neutral pH is necessary. The design of fluorescent sensors for Hg2+ has attracted tremendous attention since they facilitate easy, high sensitivity, rapid detection at low cost by simple fluorescence analysis.5 Currently, although many types of Hg2+ ion-sensing sensors have been introduced to detect Hg2+ and used with some success in biological applications, most of the reported sensors have certain shortcomings such as inefficient selectivity,6 poor detectivity,7 slow response or poor solubility in aqueous media.8 Therefore, more sensors are still needed for the analysis of Hg2+ ion in various types of sample.In order to develop a novel and efficient chemosensor, the detailed sensing mechanism should be thoroughly understood. Among the known sensing mechanisms, ligand-to-metal charge transfer (LMCT) has seldom been reported, although it is a classic mechanism in inorganic coordination compounds. The few reported quenching-type sensors based on the LMCT mechanism have been focused on Cu2+ ion.9 Usually, Hg2+ ion causes fluorescence quenching upon binding with fluorescent sensors owing to spin–orbit coupling, electron transfer and energy transfer.10 However, the mechanistic details of the quenching response induced by Hg2+ are unclear in most cases. To date, reports on Hg2+-induced fluorescence quenching based on LMCT are rare. To the best of our knowledge, only one article has mentioned that fluorescence quenching of receptor binding to Hg2+ is caused by the LMCT mechanism, but there is no further proof.11 Hence, the detailed photophysical studies of the quenching response will be valuable for future work.
In this work, we synthesized a new water-soluble fluorescent sensor (L) (Scheme 1). Sensor L exhibits highly selective and sensitive recognition of Hg2+ in aqueous solution through fluorescence “ON–OFF” behavior. The detailed binding mode of L with Hg2+ was disclosed by single-crystal X-ray diffraction analysis of the L–Hg2+ complex. Quantum calculation results revealed that the Hg2+-induced fluorescence “ON–OFF” response of L can be attributed to the LMCT effect.
Results and discussion
Synthesis, characterization and fluorescent responses of ligand L
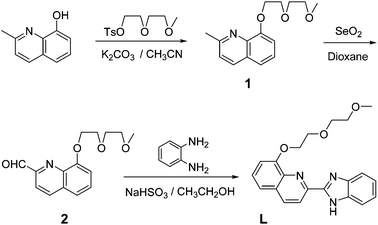
Sensor L was synthesized following the procedures as outlined in Scheme 1. The combination of 8-hydroxyquinoline (8-HQ) and the benzimidazole moiety as the fluorophore is based on their respective excellent photophysical properties.12 A combination of both moieties can provide more binding sites and improve the fluorescence properties. The diglycol monomethyl ether introduced to quinoline can act as a hydrophilic group to improve water solubility. The structure of L was confirmed by 1H nuclear magnetic resonance (NMR), 13C NMR, high-resolution mass spectrometry (HRMS) (Fig. S1–S3, ESI†) and single-crystal X-ray diffraction analysis.A single crystal, suitable for X-ray diffraction, was obtained by slow evaporation of a dichloromethane solution of L. Single-crystal X-ray diffraction analysis revealed that ligand L crystallizes in an orthorhombic crystal system with a Pbcn space group (Fig. 1). There is one ligand L molecule in the crystallography independent asymmetrical cell. The skeleton formed by fragments of benzimidazole and quinoline, which are connected by ortho carbon atoms, is nearly coplanar. The three O atoms in the flexible chain and the two N atoms in the skeleton are potential chelating sites to certain metal ions.
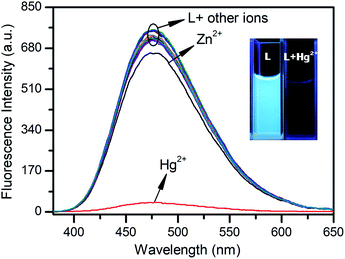
In order to examine the metal cation selectivity of L, qualitative experiments were performed by mixing L with various heavy and transition metal ions (Fig. 2). Free L solution (10 μM, 1% DMSO, Tris–HCl 10 mM, pH = 7.4) displays a strong emission at 475 nm (the quantum yield is 0.37). Upon addition of 1.0 equiv. of Hg2+, a remarkable fluorescence quenching (90%) was observed and the fluorescence color change from blue to colorless was also detectable by the naked eye (Fig. 2 inset). However, addition of 1.0 equiv. of individual cations such as Cu2+, Ag+, Pb2+, Sr2+, Ba2+, Cd2+, Ni2+, Co2+, Fe2+, Mn2+, Fe3+, Al3+, Cr3+, Mg2+, K+, and Na+ promoted no significant fluorescence changes. However, Zn2+ could induce a slight emission intensity decrease. This result indicates that L exhibits a high selectivity to Hg2+.
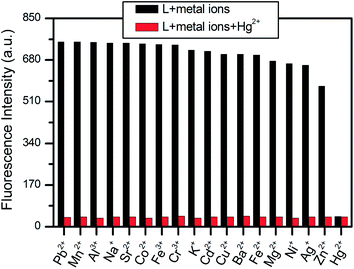
The behavior in the presence of potentially competitive metal ions was investigated by fluorescence spectroscopy. Initially, L (10 μM) was mixed with 1.0 equiv. of various metal ions (Fig. 3, black bars) followed by adding the same amount of HgCl2 (Fig. 3, red bars). Even in the presence of other metal ions, Hg2+ could still coordinate with L and cause strong fluorescence quenching. This result reveals that sensor L recognizes Hg2+ in aqueous solution without interference from the other metal ions.
To check the sensitivity of L toward Hg2+, fluorescence titration experiments were performed. Changes in fluorescence spectra of L (10 μM) upon addition of different amounts of Hg2+ ion are shown in Fig. 4. The fluorescence intensity of L solution gradually decreased with stepwise increases in the added Hg2+ concentration (0 to 1.0 equiv.). Further increasing of the Hg2+ concentration did not induce a significant fluorescence emission change, indicating a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation of L with Hg2+. A Job plot further indicated that L chelated with Hg2+ at 1
1 complexation of L with Hg2+. A Job plot further indicated that L chelated with Hg2+ at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
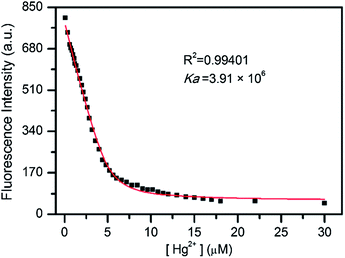
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry (Fig. S4, ESI†). Based on the titration profiles, the association constant (Ka) of L and Hg2+ was calculated to be 3.91 × 106 M−1 by nonlinear least-squares fitting (R2 = 0.99401) using the 1
1 stoichiometry (Fig. S4, ESI†). Based on the titration profiles, the association constant (Ka) of L and Hg2+ was calculated to be 3.91 × 106 M−1 by nonlinear least-squares fitting (R2 = 0.99401) using the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding equation (Fig. 5).13 In addition, a time course study revealed that the Hg2+-induced fluorescence quenching can complete within 1 minute (Fig. S5, ESI†), indicating the rapid response of sensor L to Hg2+. Moreover, on addition of excess ethylenediamine tetraacetic acid disodium salt (EDTANa2) to L–Hg2+ solution, the fluorescence signal of the resulting solution restored to that of free L (Fig. S6, ESI†), indicating that the Hg2+ recognition event is reversible. All these fluorescence study results reveal that sensor L behaves with good sensitivity toward Hg2+ and can rapidly and reversibly recognize Hg2+ in aqueous solution.
1 binding equation (Fig. 5).13 In addition, a time course study revealed that the Hg2+-induced fluorescence quenching can complete within 1 minute (Fig. S5, ESI†), indicating the rapid response of sensor L to Hg2+. Moreover, on addition of excess ethylenediamine tetraacetic acid disodium salt (EDTANa2) to L–Hg2+ solution, the fluorescence signal of the resulting solution restored to that of free L (Fig. S6, ESI†), indicating that the Hg2+ recognition event is reversible. All these fluorescence study results reveal that sensor L behaves with good sensitivity toward Hg2+ and can rapidly and reversibly recognize Hg2+ in aqueous solution.
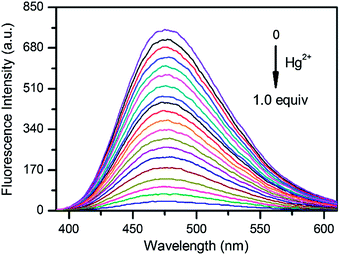 | ||
| Fig. 4 Fluorescence emission spectra of L (10 μM) upon addition of different amounts of Hg2+ (0 to 1.0 equiv.) in aqueous solution (1% DMSO, Tris–HCl 10 mM, pH = 7.4, λex = 348 nm). | ||
The binding mode of L with Hg2+ and the fluorescence quenching mechanism
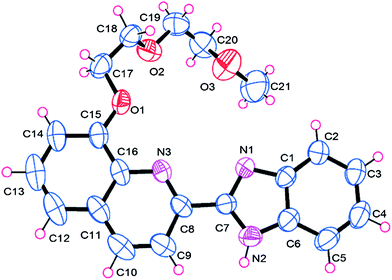
To obtain clear insight into the binding mode of L with Hg2+, an X-ray diffraction study of the L–Hg2+ complex was performed. The single crystal of L–Hg2+ was obtained by slow evaporation of the solution of L in methanol in the presence of 1.0 equiv. of HgCl2. The complex crystallizes in the triclinic system, with space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) and representing 1
and representing 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry between L and Hg2+ (Fig. 6). There is one ligand L, one Hg2+, and two Cl− anions in the crystallographically independent asymmetrical cell. The Hg2+ is five-coordinated by two N atoms from the aromatic skeleton, one O atom from the flexible chain, and two Cl− anions (Hg–O1 2.788 Å, Hg–N1 2.315 Å, Hg–N3 2.480 Å, Hg–Cl1 2.384 Å, Hg–Cl2 2.385 Å), which exhibits irregular pyramid geometrical configuration.
1 stoichiometry between L and Hg2+ (Fig. 6). There is one ligand L, one Hg2+, and two Cl− anions in the crystallographically independent asymmetrical cell. The Hg2+ is five-coordinated by two N atoms from the aromatic skeleton, one O atom from the flexible chain, and two Cl− anions (Hg–O1 2.788 Å, Hg–N1 2.315 Å, Hg–N3 2.480 Å, Hg–Cl1 2.384 Å, Hg–Cl2 2.385 Å), which exhibits irregular pyramid geometrical configuration.
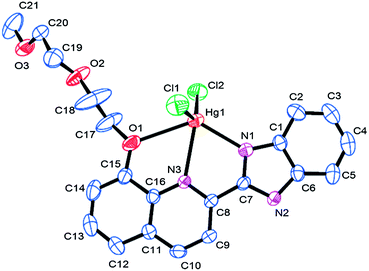 | ||
| Fig. 6 Crystal structure of L–Hg2+ with displacement atomic ellipsoids drawn at the 30% probability level. All hydrogen atoms are omitted for clarity. | ||
To clarify the mechanism of the fluorescence quenching by the energy or charge transfer model, we carried out the orbital calculations using the method of PBE0 in time-dependent density functional theory (TD-PBE0). The fluorescence quenching by Hg2+ could be rationalized in terms of orbital transition for the lowest energy excitation. According to the TD-PBE0 results (Fig. 7), the HOMO of L–Hg2+ is predominantly localized on the benzimidazole and quinoline moieties, whereas, its LUMO is mainly localized on the two N atoms and O atoms coordinated with Hg2+. The lowest energy excitation is contributed by HOMO to LUMO+2 energy excitation of configuration coefficient 91%. These excitations correspond to charge transfer from the excited L moiety to the Hg2+ center (LMCT) and, thus, provide a main pathway for nonradiative deactivation of the excited state.
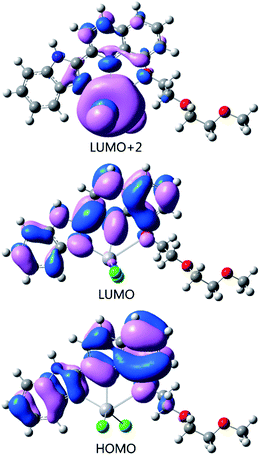 | ||
| Fig. 7 Frontier molecular orbitals of L–Hg2+ relevant to the fluorescence quenching, by the TD-PBE0 method. | ||
Determination of the detection limit and effect of pH
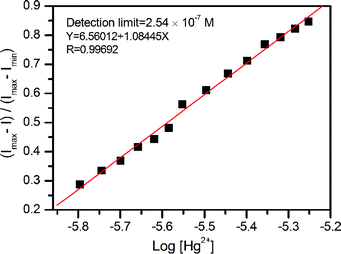
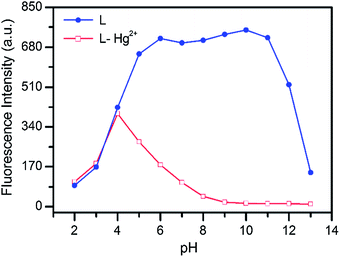
To check its practical utility, the fluorescence detection limit of L for Hg2+ and the proper pH condition were evaluated. Based on the results of the fluorescence titration experiment, the detection limit was calculated to be 2.54 × 10−7 M (Fig. 8), which is comparable to those of some previously reported Hg2+-selective sensors with similar structure to L.8d,14Moreover, the acid–base titration experiments showed that the fluorescence intensity of L almost remains constant across the pH range from 5 to 11 (Fig. 9). However, in the presence of the Hg2+, there was an obvious fluorescence “ON–OFF” change between pH 5 and 13. Thus, sensor L can detect Hg2+ ion over a wide pH range from 5 to 11. This result suggests that buffer solutions are not necessary for the detection of Hg2+ ion and sensor L has potential applicability in environmental monitoring work.
Conclusions
We have developed a new water-soluble fluorescent sensor L, based on 2-(2-(8-hydroxyquinolin)-yl)benzimidazole, which exhibits highly selective, sensitive and quick recognition of Hg2+ with a fluorescence “ON–OFF” response in aqueous solution (1% DMSO, Tris–HCl 10 mM, pH = 7.4). The X-ray crystal structure of the L–Hg2+ complex reveals that the oxygen and nitrogen atoms from the 8-hydroxyquinoline moiety, together with the nitrogen atom from the benzimidazole unit (N1) bind Hg2+ through a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry. The density functional theory (DFT) calculations demonstrate that the fluorescence quenching phenomenon is caused by LMCT. The low limit of detection and a wide usable pH range from 5 to 11 make it potentially applicable for determining Hg2+ ions in aqueous solution.
1 binding stoichiometry. The density functional theory (DFT) calculations demonstrate that the fluorescence quenching phenomenon is caused by LMCT. The low limit of detection and a wide usable pH range from 5 to 11 make it potentially applicable for determining Hg2+ ions in aqueous solution.
Experimental
Instruments and materials
All reagents and other chemicals were obtained from Aladdin Industrial Corporation (Shanghai, China) and used without further purification. NMR spectra were recorded on an Agilent 400-MR NMR spectrometer (Agilent, USA). Chemical shifts (δ) were expressed in parts per million (ppm) and coupling constants (J) in hertz. HRMS was measured on an Agilent 1200 Series Binary SL HPLC/Bruker micrOTOF mass spectrometer (Bruker, Germany). Fluorescence measurements were performed on a Sanco 970-CRT spectrofluorometer (Shanghai, China) and the excitation and emission slit widths were 2 nm. The pH measurements were made with a Model PHS-25B meter (Shanghai, China).The salts used in stock solutions of metal ions were Ni(NO3)2·6H2O, HgCl2, Ba(NO3)2, Mg(NO3)2·6H2O, AgNO3, FeSO4·7H2O, KNO3, Al(NO3)3·9H2O, Mn(NO3)2, Pb(NO3)2, NaNO3, Sr(NO3)2, Cu(NO3)2·3H2O, Co(NO3)2·6H2O, Cd(NO3)2, Zn(NO3)2·6H2O, CrCl3·6H2O and Fe(NO3)3·9H2O.
General methods
All titration experiments were carried out at room temperature. Fluorescence spectra were measured with a 10 μM solution of sensor L in buffered water solution (1% DMSO, Tris–HCl 10 mM, pH = 7.4) by excitation at 348 nm. The solutions of metal salts were prepared in distilled water. These solutions were used for all spectroscopic studies after appropriate dilution. Double-distilled water was used throughout the experiments. The fluorescence spectra were measured 3 minutes after metal ion addition.Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as the eluent to obtain a light brown solid 1 (3.85 g, 74%). 1H NMR (400 MHz, CDCl3) δ 8.00 (d, J = 8.4 Hz, 1H), 7.42–7.32 (m, 2H), 7.29 (d, J = 8.4 Hz, 1H), 7.10 (d, J = 6.7 Hz, 1H), 4.44 (t, J = 5.6 Hz, 2H), 4.08 (t, J = 5.6 Hz, 2H), 3.81 (t, 2H), 3.60 (t, 2H), 3.40 (s, 3H), 2.77 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 158.07, 153.87, 139.54, 136.14, 127.63, 125.62, 122.53, 119.73, 109.13, 71.83, 70.73, 69.42, 67.86, 59.07, 25.38.
1, v/v) as the eluent to obtain a light brown solid 1 (3.85 g, 74%). 1H NMR (400 MHz, CDCl3) δ 8.00 (d, J = 8.4 Hz, 1H), 7.42–7.32 (m, 2H), 7.29 (d, J = 8.4 Hz, 1H), 7.10 (d, J = 6.7 Hz, 1H), 4.44 (t, J = 5.6 Hz, 2H), 4.08 (t, J = 5.6 Hz, 2H), 3.81 (t, 2H), 3.60 (t, 2H), 3.40 (s, 3H), 2.77 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 158.07, 153.87, 139.54, 136.14, 127.63, 125.62, 122.53, 119.73, 109.13, 71.83, 70.73, 69.42, 67.86, 59.07, 25.38.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as the eluent to obtain 2 as yellow liquid (2.2 g, 80%). 1H NMR (400 MHz, CDCl3) δ 10.28 (s, 1H), 8.28 (d, J = 5.2 Hz, 1H), 8.06 (d, J = 5.2 Hz, 1H), 7.61 (t, J = 5.2 Hz, 1H), 7.48 (d, 1H), 7.21 (d, J = 5.2 Hz, 1H), 4.49 (t, 2H), 4.13 (t, 2H), 3.84 (t, 2H), 3.61 (t, 2H), 3.40 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 193.93, 155.48, 151.35, 140.09, 137.30, 131.54, 129.94, 119.81, 117.76, 110.13, 71.92, 71.00, 69.40, 68.73, 59.06.
1, v/v) as the eluent to obtain 2 as yellow liquid (2.2 g, 80%). 1H NMR (400 MHz, CDCl3) δ 10.28 (s, 1H), 8.28 (d, J = 5.2 Hz, 1H), 8.06 (d, J = 5.2 Hz, 1H), 7.61 (t, J = 5.2 Hz, 1H), 7.48 (d, 1H), 7.21 (d, J = 5.2 Hz, 1H), 4.49 (t, 2H), 4.13 (t, 2H), 3.84 (t, 2H), 3.61 (t, 2H), 3.40 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 193.93, 155.48, 151.35, 140.09, 137.30, 131.54, 129.94, 119.81, 117.76, 110.13, 71.92, 71.00, 69.40, 68.73, 59.06.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, v/v) as the eluent to give 175 mg (24%) of L as a fulvous solid. 1H NMR (400 MHz, CDCl3) δ 12.87 (s, 1H), 8.62 (d, J = 8.6 Hz, 1H), 8.27 (d, J = 8.6 Hz, 1H), 7.83 (d, J = 7.9 Hz, 1H), 7.44 (d, J = 4.4 Hz, 2H), 7.27–7.05 (m, 4H), 4.31 (t, J = 4.5 Hz, 2H), 3.83 (t, J = 4.5 Hz, 2H), 3.58 (m, 2H), 3.47 (m, 2H), 3.34 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 154.56, 151.22, 147.77, 144.48, 139.54, 136.99, 134.73, 129.66, 127.04, 123.52, 121.91, 119.94, 119.60, 111.84, 109.78, 71.80, 70.49, 69.21, 68.34, 58.77. HRMS (ESI†) m/z, calculated for C21H21N3O3 [M + H]+: 364.1583; found: 364.1656.
10, v/v) as the eluent to give 175 mg (24%) of L as a fulvous solid. 1H NMR (400 MHz, CDCl3) δ 12.87 (s, 1H), 8.62 (d, J = 8.6 Hz, 1H), 8.27 (d, J = 8.6 Hz, 1H), 7.83 (d, J = 7.9 Hz, 1H), 7.44 (d, J = 4.4 Hz, 2H), 7.27–7.05 (m, 4H), 4.31 (t, J = 4.5 Hz, 2H), 3.83 (t, J = 4.5 Hz, 2H), 3.58 (m, 2H), 3.47 (m, 2H), 3.34 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 154.56, 151.22, 147.77, 144.48, 139.54, 136.99, 134.73, 129.66, 127.04, 123.52, 121.91, 119.94, 119.60, 111.84, 109.78, 71.80, 70.49, 69.21, 68.34, 58.77. HRMS (ESI†) m/z, calculated for C21H21N3O3 [M + H]+: 364.1583; found: 364.1656.X-ray crystal structure determination
The X-ray diffraction data were collected under a nitrogen stream at 293 K on a Bruker D8 SMART APEX CCD single-crystal diffractometer with graphite-monochromated Mo Kα (λ = 0.71073 Å) radiation. Data collection, indexing, and initial cell refinements were processed using the SMART software while frame integration and final cell refinements were carried out using the SAINT software. The final cell parameters were determined from the least-squares refinement of total reflections. The structures were determined through direct methods (SHELXL97) and difference Fourier maps (SHELXL97). The final results of crystal data and structure refinement are summarized in Table S1 (ESI†).DFT calculations
DFT calculations15 with hybrid-type Perdew–Burke–Ernzerhof exchange correlation functional (PBE0).16 In calculations, a “double-ξ” quality basis set LANL2DZ17 associated with the pseudopotential was employed on the mercury atom and the 6-31G*18 basis set was used on C, H, N, Cl and O atoms. All calculations were performed with the Gaussian 09 package.Acknowledgements
This work is supported by the National Natural Science Foundation of China (no. 21304009, 21176029), the National Key Technologies R&D Program of China during the 12th Five-Year Plan Period (no. 2012BAD29B06), Food Safety Key Laboratory of Liaoning Province (no. LNSAKF2011025), the Program for Liaoning Excellent Talents in University (LJQ2012096) for financial support.Notes and references
- W. F. Fitzgerald, C. H. Lamborg and C. R. Hammerschmidt, Chem. Rev., 2007, 107, 641 CrossRef CAS PubMed.
- (a) R. K. Zalups and S. Ahmad, J. Am. Soc. Nephrol., 2004, 15, 2023 CrossRef CAS PubMed; (b) R. K. Zalups and L. H. Lash, Toxicol. Appl. Pharmacol., 2006, 214, 88 CrossRef CAS PubMed; (c) E. K. Silbergeld, I. A. Silva and J. F. Nyland, Toxicol. Appl. Pharmacol., 2005, 207, S282 CrossRef CAS PubMed.
- I. Onyido, A. R. Norris and E. Buncel, Chem. Rev., 2004, 104, 5911 CrossRef CAS PubMed.
- P. B. Tchounwou, W. K. Ayensu, N. Ninashvili and D. Sutton, Environ. Toxicol., 2003, 18, 149 CrossRef CAS PubMed.
- (a) Z. Liu, W. He and Z. Guo, Chem. Soc. Rev., 2013, 42, 1568 RSC; (b) H. N. Kim, W. X. Ren, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2012, 41, 3210 RSC; (c) M. Dutta and D. Das, TrAC, Trends Anal. Chem., 2012, 32, 113 CrossRef CAS PubMed; (d) D. T. Quang and J. S. Kim, Chem. Rev., 2010, 110, 6280 CrossRef CAS PubMed; (e) E. M. Nolan and S. J. Lippard, Chem. Rev., 2008, 108, 3443 CrossRef CAS PubMed; (f) B. Valeur and I. Leray, Coord. Chem. Rev., 2000, 205, 3 CrossRef CAS.
- L. N. Neupane, J. M. Kim, C. R. Lohani and K.-H. Lee, J. Mater. Chem., 2012, 22, 4003 RSC.
- Y. Yu, L.-R. Lin, K.-B. Yang, X. Zhong, R.-B. Huang and L.-S. Zheng, Talanta, 2006, 69, 103 CrossRef CAS PubMed.
- (a) R. Métivier, I. Leray and B. Valeur, Chem.–Eur. J., 2004, 10, 4480 CrossRef PubMed; (b) Q.-Y. Chen and C.-F. Chen, Tetrahedron Lett., 2005, 46, 165 CrossRef CAS PubMed; (c) J. H. Kim, A.-R. Hwang and S.-K. Chang, Tetrahedron Lett., 2004, 45, 7557 CrossRef CAS PubMed; (d) Y.-H. Kim, J. S. Youk, S. Y. Moon, J.-I. Choe and S.-K. Chang, Chem. Lett., 2004, 33, 702 CrossRef CAS.
- (a) H. S. Jung, P. S. Kwon, J. W. Lee, J. I. Kim, C. S. Hong, J. W. Kim, S. Yan, J. Y. Lee, J. H. Lee and T. Joo, J. Am. Chem. Soc., 2009, 131, 2008 CrossRef CAS PubMed; (b) D. Maity, A. K. Manna, D. Karthigeyan, T. K. Kundu, S. K. Pati and T. Govindaraju, Chem.–Eur. J., 2011, 17, 11152 CrossRef CAS PubMed; (c) Q. Li, Y. Guo and S. Shao, Sens. Actuators, B, 2012, 171–172, 872 CrossRef CAS PubMed.
- (a) J. C. Koziar and D. O. Cowan, Acc. Chem. Res., 1978, 11, 334 CrossRef CAS; (b) P. Svejda, A. H. Maki and R. R. Anderson, J. Am. Chem. Soc., 1978, 100, 7138 CrossRef CAS; (c) H. Masuhara, H. Shioyama, T. Saito, K. Hamada, S. Yasoshima and N. Mataga, J. Phys. Chem., 1984, 88, 5868 CrossRef CAS; (d) C. N. Burress, M. I. Bodine, O. Elbjeirami, J. H. Reibenspies, M. A. Omary and F. P. Gabbaï, Inorg. Chem., 2007, 46, 1388 CrossRef CAS PubMed.
- Y. Yu, W. Dou, X. Hu, X. Tang, X. Zhou and W. Liu, J. Fluoresc., 2012, 22, 1547 CrossRef CAS PubMed.
- (a) N. R. Chereddy, S. Thennarasu and A. B. Mandal, Dalton Trans., 2012, 41, 11753 RSC; (b) J.-S. Youk, Y. H. Kim, E.-J. Kim, N. J. Youn and S.-K. Chang, Bull. Korean Chem. Soc., 2004, 25, 869 CrossRef CAS; (c) R. Ohshima, M. Kitamura, A. Morita, M. Shiro, Y. Yamada, M. Ikekita, E. Kimura and S. Aoki, Inorg. Chem., 2010, 49, 888 CrossRef CAS PubMed; (d) H. Zhu, J. Fan, J. Lu, M. Hu, J. Cao, J. Wang, H. Li, X. Liu and X. Peng, Talanta, 2012, 93, 55 CrossRef CAS PubMed; (e) L. Tang, N. Wang, Q. Zhang, J. Guo and R. Nandhakumar, Tetrahedron Lett., 2013, 54, 536 CrossRef CAS PubMed; (f) H. J. Kim, C. H. Heo and H. M. Kim, J. Am. Chem. Soc., 2013, 135, 17969 CrossRef CAS PubMed; (g) M. Wang, J. Wang, W. Xue and A. Wu, Dyes Pigm., 2013, 97, 475 CrossRef CAS PubMed; (h) L. J. Tang, M. J. Cai, Z. L. Huang, K. L. Zhong, S. H. Hou, Y. J. Bian and R. Nandhakumar, Sens. Actuators, B, 2013, 185, 188 CrossRef CAS PubMed.
- H. Gampp, M. Maeder, C. J. Meyer and A. D. Zuberbühler, Talanta, 1985, 32, 1133 CrossRef CAS.
- K. C. Song, J. S. Kim, S. M. Park, K.-C. Chung, S. Ahn and S.-K. Chang, Org. Lett., 2006, 8, 3413 CrossRef CAS PubMed.
- E. Runge and E. K. Gross, Phys. Rev. Lett., 1984, 52, 997 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS.
- P. J. Hay and W. R. Wadt, J. Chem. Phys., 1985, 82, 299 CrossRef CAS PubMed.
- P. C. Hariharan and J. A. Pople, Mol. Phys., 1974, 27, 209 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: NMR and HRMS spectra of sensor L, crystal data and supplementary spectra. CCDC 971509 and 971510. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra00060a |
| This journal is © The Royal Society of Chemistry 2014 |