Magnetic scaffolds of polycaprolactone with functionalized magnetite nanoparticles: physicochemical, mechanical, and biological properties effective for bone regeneration
Abstract
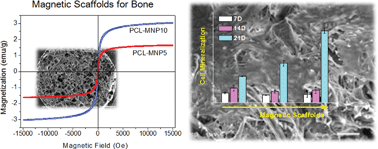
Magnetic scaffolds have gained significant attention for disease treatment and tissue repair. Here we focus on magnetic nanocomposite scaffolds made of poly(caprolactone) (PCL) and magnetite nanoparticles (MNPs) for bone repair. The physico-chemical, mechanical, and magnetic properties of the scaffolds, the in vitro cell responses, and the in vivo tissue compatibility were examined in-depth to investigate their effectiveness for use as bone scaffolds. The MNPs, produced by a surfactant-mediation process, were well-distributed within the PCL matrix to enable homogeneous nanocomposites. The PCL–MNP scaffolds showed excellent magnetic properties, preserving the superparamagnetic behavior. Incorporation of MNPs greatly improved the hydrophilicity and water swelling of scaffolds. Acellular apatite forming ability tests revealed a higher mineral induction on the magnetic scaffolds than on the PCL scaffold. The mechanical stiffness increased significantly with the addition of MNPs, when tested under both static and dynamic compressed wet conditions. The initial cell adhesion to the magnetic scaffolds was substantially improved by ∼1.4-fold with respect to the pure PCL scaffold, enabling earlier cellular proliferation confluence. The cell mineralization, as assessed by the quantification of calcium deposits, was significantly enhanced on the magnetic scaffolds. The magnetic scaffolds, subcutaneously implanted in rats for 2 weeks, revealed favorable tissue compatibility, with substantial fibroblastic cell invasion and neoblood vessel formation while exerting minimal inflammatory reactions. The results, demonstrating excellent physico-chemical, magnetic, mechanical and biological properties of the PCL–MNP scaffolds, support the potential use of the magnetic scaffolds for bone repair and regeneration.

 Please wait while we load your content...
Please wait while we load your content...