A Se/C composite as cathode material for rechargeable lithium batteries with good electrochemical performance†
Abstract
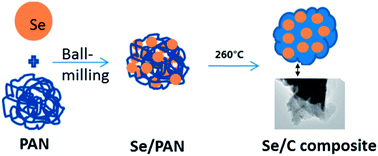
A Se/C composite was prepared by a simple combination method of ball milling and low temperature treatment as a cathode material for Li–Se rechargeable batteries. It was characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Its electrochemical performance as a cathode material for lithium rechargeable batteries was tested by cyclic voltammetry (CV) and capacity measurements. Rate capacity and cycling performance of the as-prepared product are very satisfactory. Even at a current density of 500 mA g−1, the composite can deliver a capacity of 187 mA h g−1. The main reason is that the high conductivity of carbon decreases its charge transfer resistance and effectively suppresses the dissolution of oxidation products from the composite cathode.

 Please wait while we load your content...
Please wait while we load your content...