Chitin hybrid materials reinforced with graphene oxide nanosheets: chemical and mechanical characterisation†
Abstract
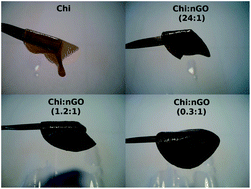
Chitin hybrid materials reinforced with graphene oxide nanosheets (nGO) have been prepared. The chitin : nGO ratio ranged from proportions where chitin was the main component to ones where nGO exceeded chitin. SEM and TEM images showed that high proportions of nGO may result in nanosheet association. FTIR, 13C solid-state NMR and DSC analyses showed that the interaction among the components would not involve the formation of new molecular bonds. nGO was shown to act as a filler that induces structural rearrangements in chitin which lead to new hydrogen bonds among the chains. The mechanical stability proved to be higher when the nGO content in the hybrid was similar to or higher than that of chitin. The rheological behaviour of the material was shown to become more solid-like with increasing nGO content. The nGO did not interfere with lysozyme activity on chitin chains, indicating that these materials would be biodegradable.

 Please wait while we load your content...
Please wait while we load your content...