Mixed-solvent strategy for solvothermal synthesis of well-dispersed YBO3:Ce3+,Tb3+ nanocrystals†
Akihiro Nohara,
Satoru Takeshita* and
Tetsuhiko Isobe*
Department of Applied Chemistry, Faculty of Science and Technology, Keio University, 3-14-1 Hiyoshi, Kohoku-ku, Yokohama 223-8522, Japan. E-mail: takeshita@applc.keio.ac.jp; isobe@applc.keio.ac.jp; Fax: +81 45 566 1551; Tel: +81 45 566 1531
First published on 11th February 2014
Abstract
We investigated the influence of the composition of a 1,4-butanediol–water mixed solvent on the structural and particulate properties in the synthesis of well-dispersed YBO3:Ce3+,Tb3+ nanocrystals, without assembly, using a solvothermal method. YBO3:Ce3+,Tb3+ nanocrystals were synthesized from trimethyl borate and acetates of yttrium, cerium(III), and terbium(III) via a solvothermal reaction at 250 °C for 6 h in the mixed solvent. The presence of water promoted YBO3 crystallization. The size of the primary nanocrystals increased from ∼5 to ∼40 nm as the water content of the mixed solvent increased from 2.5 to 100 vol%. The primary nanocrystals formed disk-like secondary assembled particles for water contents below 75 vol%, but formed polyhedral secondary particles in pure water. In contrast, well-dispersed primary nanocrystals with a mean hydrodynamic size of ∼35 nm were obtained when the water content was 75 vol%. These results indicate that not only the size of the primary nanocrystals, but also their assembly can be controlled by changing the composition of the mixed solvent. The obtained well-dispersed nanocrystals were transparent to the naked eye and showed green photoluminescence corresponding to 4f → 4f transitions of Tb3+ by energy transfer from Ce3+ to Tb3+ under near-UV irradiation.
1 Introduction
Inorganic luminescent materials have been widely used in various optical, optoelectronic, and biological devices, such as white-light-emitting diodes,1–4 displays,5 and biological labels,6–9 because of their high durability and thermal and chemical stabilities. Phosphors emitting visible colors under near-UV excitation are required for wavelength conversion in high-color-rendering white-light-emitting diodes10–12 and spectral downshifters for solar cells.13,14 Control of the phosphor particle size is essential for efficient wavelength conversion in these devices. However, most commercial phosphors are of micrometer size, and cannot be used in devices that require high transparency in the visible region because of their light scattering. On the basis of Rayleigh's law, the light-scattering intensity from a small particle is proportional to the particle size to the sixth power.15 We need well-dispersed nanoparticles of size less than ∼50 nm to achieve high transparency to the naked eye, i.e., negligible light-scattering intensity in the visible region.16 Moreover, each nanoparticle should be a single crystal, i.e., a nanocrystal, without crystallite boundaries, because defects near the boundaries act as luminescence quenchers.Vaterite-type YBO3 has high thermal and chemical stabilities, an exceptionally high optical damage threshold, and high transparency in the near-UV and visible regions.17–19 Eu3+- and Tb3+-doped YBO3 have been exclusively studied as red- and green-emitting phosphors, respectively, for plasma display panel applications.18–22 Ce3+-doped YBO3 is an efficient blue-emitting phosphor for near-UV excitation.23–25 We recently reported that Ce3+- and Tb3+-codoped YBO3 prepared using a solid-state method emitted green light under near-UV irradiation through 4f1 → 5d1 transitions of Ce3+ followed by energy transfer from Ce3+ to Tb3+.26 We also reported that the optimum concentrations were 3 at% for Ce3+ and 15 at% for Tb3+.
Various chemical methods for rare-earth-doped YBO3 synthesis such as solid-state reactions,26–29 coprecipitation methods,30,31 sol–gel methods,30,32,33 combustion methods,34 splay pyrolysis,21,35 and solvothermal methods,17–19,23,25,36–43 have been reported. Despite the range of available synthetic methods, most previous reports have dealt with micrometer-sized particles. A number of researchers have reported syntheses of micrometer- and submicrometer-sized YBO3 particles using hydrothermal methods.17,19,23,25,37,39,41 Hosokawa et al. reported a glycothermal method, i.e., a solvothermal method using glycols as solvents, for the preparation of monodispersed rare-earth borate particles of several micrometers in size.36 We prepared disk-like assembled particles of YBO3:Ce3+ by the glycothermal method using 1,4-butanediol as the solvent.43 We also reported that Ce3+ doping of YBO3 crystals decreased the size of the assembled nanoparticles from ∼1 μm to ∼180 nm.44 However, this size range was not small enough to achieve high transparency in the visible region, and the glycothermal method did not produce well-dispersed YBO3 nanocrystals without assembly. It is still necessary to develop a novel chemical method for preparing well-dispersed YBO3 nanocrystals with a controllable size of less than 50 nm.
Solvothermal methods using mixed glycol/water solvents are suitable routes for the preparation of size-controlled inorganic materials. Jung et al. reported a solvothermal synthesis of BaTiO3 nanoparticles in a mixed 1,4-butanediol–water solvent.45 We reported a size-tunable solvothermal synthesis of Zn2GeO4:Mn2+ nanophosphors using mixed solvents containing different ratios of diethylene glycol (DEG) and water.46 In that previous report, the length of the Zn2GeO4:Mn2+ nanorods decreased from ∼120 to ∼30 nm as the DEG content increased from 0 to 91.7 vol%. Although the size-tunability mechanism was complicated, we suggested that the overall polarity of the solvent played a significant role in crystal growth; a high ratio of DEG to water reduced the solute solubilities and therefore suppressed crystal growth, resulting in small particles.
The purpose of the present work is to establish a novel low-temperature route for the preparation of well-dispersed YBO3:Ce3+,Tb3+ nanocrystals of size less than 50 nm. We focused on a solvothermal method using a 1,4-butanediol–water mixed solvent with various water volume fractions and investigated the influence of the solvent composition on particle size and assembly. We also investigated the photoluminescence (PL) properties of the nanocrystals.
2 Experimental section
2.1 Sample preparation
A mixed solvent (20 mL) consisting of 1,4-butanediol (Kanto Chemical, 97.0%) and ultrapure water was poured into a Teflon-lined stainless-steel autoclave (Berghof, DAB-2, inner volume 50 mL) and purged with Ar gas for 30 min at a flow rate of 300 mL min−1. Yttrium acetate tetrahydrate (1.64 mmol, Kanto Chemical, 99.99%), cerium(III) acetate monohydrate (0.06 mmol, Kanto Chemical, 99.99%), terbium(III) acetate tetrahydrate (0.30 mmol, Wako, 99.9%), and trimethyl borate (2.00 mmol, Sigma-Aldrich, 98%) were added to the mixed solvent. The nominal Y–Ce–Tb composition was 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 (at%) and the nominal molar ratio of B/(Y + Ce + Tb) was 1/1. The autoclave was sealed, heated to 250 °C, and kept at that temperature for 6 h. After cooling to room temperature, ethanol (40 mL) was added to the suspension and the precipitate was collected by centrifugation at 13
15 (at%) and the nominal molar ratio of B/(Y + Ce + Tb) was 1/1. The autoclave was sealed, heated to 250 °C, and kept at that temperature for 6 h. After cooling to room temperature, ethanol (40 mL) was added to the suspension and the precipitate was collected by centrifugation at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min. The supernatant was carefully removed and the precipitate was washed with ethanol and centrifuged at 13
000 rpm for 30 min. The supernatant was carefully removed and the precipitate was washed with ethanol and centrifuged at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min. This washing process was repeated twice. Finally, the precipitate was dried at 50 °C for 1 day, giving a powdered sample. The volume percentage of water in the mixed solvent, x, ranged from 0 to 100 vol%. The samples were denoted by Wx. We also prepared Ce3+-monodoped and Tb3+-monodoped YBO3 samples with x = 75 vol% for comparison.
000 rpm for 15 min. This washing process was repeated twice. Finally, the precipitate was dried at 50 °C for 1 day, giving a powdered sample. The volume percentage of water in the mixed solvent, x, ranged from 0 to 100 vol%. The samples were denoted by Wx. We also prepared Ce3+-monodoped and Tb3+-monodoped YBO3 samples with x = 75 vol% for comparison.
2.2 Characterization
Powder X-ray diffraction (XRD) patterns were obtained using an X-ray diffractometer (Rigaku, Rint-2200) with a Cu Kα radiation source and a monochromator. The crystallite sizes perpendicular to the (100) and (002) planes of the hexagonal YBO3 crystals, D(100) and D(002), were calculated from the full widths at half maximum of the respective XRD peaks using Scherrer's equation. The metallic contents of the powdered samples were determined by the fundamental parameter method using an X-ray fluorescence analyzer (Rigaku, ZSXmini II). The particle size and morphology were determined using field-emission transmission electron microscopy (TEM; FEI, Tecnai 12 and Tecnai G2). The samples for TEM observations were prepared by dropping an aqueous dispersion onto a copper microgrid and drying at 50 °C. Fourier-transform infrared (FT-IR) spectra were recorded using the diffuse reflection method (JASCO, FT/IR-4200). The thermal behaviors of the powdered samples were investigated using thermogravimetry and differential thermal analysis (TG-DTA; Rigaku, Thermo Plus TG 8120) in an air flow of 300 mL min−1 at a heating rate of 10 °C min−1, with α-Al2O3 as a reference. The specific surface areas of the powdered samples were determined by the Brunauer–Emmett–Teller nitrogen adsorption method, using an automatic surface area analyzer (Micromeritics, Tristar II 3020). Hydrodynamic size distributions were measured using dynamic light-scattering (DLS; Malvern, HPPS); the colloidal solution was diluted in 1,4-butanediol and the refractive index of bulk YBO3, 1.788, was used. The PL spectra, PL excitation (PLE) spectra, and decay curves were obtained using a fluorescence spectrometer (JASCO, FP-6500) with a 150 W Xe lamp. The spectral response was calibrated using an ethylene glycol solution of rhodamine B (5.5 g L−1) and a standard light source (JASCO, ESC-333). An integrating sphere (JASCO, ISF-513) was used to measure the PL quantum efficiency, Φ, defined by the following equation:
 | (1) |
3 Results and discussion
3.1 Influence of water content on structure and composition
Fig. 1 shows the XRD profiles of the samples prepared in the mixed solvent at different water contents. All the XRD peaks are from YBO3 with the hexagonal vaterite structure, irrespective of the water content. The XRD peaks of sample W0 are broader and weaker than those of the other samples. This indicates a lower degree of crystallization, i.e., the presence of an amorphous phase. The XRD peaks of sample W2.5 are about eight times stronger than those of W0. These results show that a small amount of water promotes YBO3 crystallization. As the data in Table 1 show, the yield of sample W0, 13.4%, is improved by up to 50–80% in the presence of water. The relatively low yield for W75, 50.3%, could be attributed to losses during washing. It is difficult to collect all the nanoparticles by centrifugation because W75 contains small, well-dispersed nanoparticles, as will be discussed in the next section. The atomic percentage of Ce/(Y + Ce + Tb) decreases from 3.5 to 1.7 at% as the water content increases from 2.5 to 100 vol%. The atomic percentage of Tb/(Y + Ce + Tb) is constant at ∼18 at%, which is slightly higher than the nominal value, irrespective of the water content. | ||
| Fig. 1 XRD profiles of powdered samples prepared at different water contents: (a) W0, (b) W2.5, (c) W25, (d) W50, (e) W75, and (f) W100. | ||
| Sample | Yield (%) | Ce/(Y + Ce + Tb) (at%) | Tb/(Y + Ce + Tb) (at%) | D(100) (nm) | D(002) (nm) |
|---|---|---|---|---|---|
| W0 | 13.4 | 8.5 | 28.9 | 20.4 | 10.7 |
| W2.5 | 71.6 | 3.5 | 18.4 | 33.1 | 20.8 |
| W25 | 83.3 | 2.5 | 16.1 | 38.7 | 38.6 |
| W50 | 66.8 | 1.9 | 15.4 | 39.1 | 35.3 |
| W75 | 50.3 | 2.0 | 17.4 | 27.7 | 27.1 |
| W100 | 64.7 | 1.7 | 17.5 | 41.7 | 38.6 |
| Nominal concentration | — | 3.0 | 15.0 | — | — |
3.2 Influence of water content on particle size and assembly
Fig. 2 and 3 show the TEM images of the samples prepared at different water contents. As shown in Fig. 2(a), sample W2.5 comprises disk-like particles of diameter 80–100 nm. Each disk-like particle consists of an assembly of small primary nanocrystals of size ∼5 nm. Clear lattice fringes corresponding to (010), (100), and (1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) of the hexagonal vaterite crystal are observed in the high-resolution TEM and fast FT (FFT) images [Fig. 3(a and b)], indicating well-crystallized YBO3 particles. These particle sizes and morphologies are similar to those of the glycothermally synthesized YBO3:Ce3+ particles described in our previous report.43 As shown in Table 1, the crystallite sizes perpendicular to the (100) and (002) planes for sample W2.5 are 33.1 and 20.8 nm, respectively. These values are larger than the primary nanocrystal size, ∼5 nm. This could be attributed to formation of mesocrystalline particles. In our previous report,43 electron diffraction profiles showed that the disk-like YBO3:Ce3+ particles were mesocrystals, i.e., all the primary nanocrystals in one disk-like particle had the same crystallographic orientation. For mesocrystalline particles, the crystallite size calculated using Scherrer's equation is larger than the primary nanocrystal size because the primary nanocrystals aggregate in a uniform crystallographic direction.
0) of the hexagonal vaterite crystal are observed in the high-resolution TEM and fast FT (FFT) images [Fig. 3(a and b)], indicating well-crystallized YBO3 particles. These particle sizes and morphologies are similar to those of the glycothermally synthesized YBO3:Ce3+ particles described in our previous report.43 As shown in Table 1, the crystallite sizes perpendicular to the (100) and (002) planes for sample W2.5 are 33.1 and 20.8 nm, respectively. These values are larger than the primary nanocrystal size, ∼5 nm. This could be attributed to formation of mesocrystalline particles. In our previous report,43 electron diffraction profiles showed that the disk-like YBO3:Ce3+ particles were mesocrystals, i.e., all the primary nanocrystals in one disk-like particle had the same crystallographic orientation. For mesocrystalline particles, the crystallite size calculated using Scherrer's equation is larger than the primary nanocrystal size because the primary nanocrystals aggregate in a uniform crystallographic direction.
 | ||
| Fig. 2 TEM images of samples prepared at different water contents: (a) W2.5, (b) W25, (c) W50, (d) W75, and (e) W100. | ||
 | ||
| Fig. 3 High-resolution TEM and FFT images of samples prepared at different water contents: (a and b) W2.5, and (c and d) W75. | ||
As shown in Fig. 2(b and c), the size of the disk-like particles decreases from ∼80 to ∼50 nm as the water content increases from 2.5 to 50 vol%. At the same time, the primary nanocrystal size becomes larger and the boundaries between the primary nanocrystals become ambiguous with increasing water content. Sample W75 comprises cubic nanocrystals of size ∼30 nm, as shown in Fig. 2(d). The uniform lattice fringes across one whole particle [Fig. 3(c and d)] show that each particle in this sample is a single crystal. Furthermore, the crystallite sizes (see Table 1) are close to the primary nanocrystal size, indicating that the primary nanocrystals are well dispersed, without mesocrystalline aggregation. In contrast, sample W100 [Fig. 2(e)] comprises polyhedral particles of diameter ∼90 nm, which consist of several coalesced primary nanocrystals of size ∼40 nm.
In summary, the primary nanocrystal size increases from ∼5 to ∼40 nm with increasing water content. This can be explained by the increasing solute solubilities in the mixed solvent with increasing water content; high solute solubilities promote dissolution–reprecipitation processes, resulting in faster growth of primary nanocrystals.46 However, these primary nanocrystals form assembled particles when the water content is smaller or larger than 75 vol%. The FT-IR spectra and TG-DTA profiles (Fig. S1 and S2 in the ESI†) show that the particle surfaces are covered with 1,4-butanediol and acetate ions. The amounts of these organic species mainly depend on the specific surface area (see Fig. S3 in the ESI†). We suggest that surface-attached 1,4-butanediol molecules play a significant role in assembly of the primary nanocrystals. In the absence of 1,4-butanediol, the primary nanocrystals form secondary assembled particles, because of the high surface energy, as observed for sample W100. In the presence of a certain amount of 1,4-butanediol, the primary nanocrystals are well dispersed, as a result steric repulsion between surface-attached 1,4-butanediol molecules, as observed for sample W75. In the presence of a large amount of 1,4-butanediol, the primary nanocrystals also form assemblies, although the mechanism has not yet been clarified. On the basis of the discussion in our previous report, the YBO3 mesocrystals are probably formed via self-organized growth; repeated growth and growth inhibition caused by the adsorption and desorption of organic molecules at the nanocrystal surfaces result in a spatially periodic assembly of bridged nanocrystals.43,47 Further investigation is needed to clarify the detailed mechanism of mesocrystal formation.
3.3 Influence of water content on colloidal properties
Fig. 4 shows the hydrodynamic size distributions of the samples prepared in the mixed solvent at different water contents. The mean hydrodynamic size (see circles in Fig. 5) gradually decreases from ∼80 to ∼35 nm as the water content increases from 2.5 to 75 vol%, and then increases up to ∼100 nm as the water content increases up to 100 vol%. The mean sizes of the assembled particles, measured from the TEM images, are also plotted as a function of the water content and shown as triangles in Fig. 5; they are close to the hydrodynamic size. These results show that the hydrodynamic size of YBO3:Ce3+,Tb3+ nanoparticles depends on the size of the assembled particles and can be controlled in the range 35–100 nm by changing the water content of the mixed solvent. The smallest hydrodynamic size, 35.4 ± 11.8 nm, is obtained for sample W75, which consists of well-dispersed nanocrystals. The size is small enough to achieve high transparency to the naked eye, i.e., negligible light-scattering intensity in the visible region. | ||
| Fig. 4 Hydrodynamic size distribution of samples prepared at different water contents: (a) W2.5, (b) W25, (c) W50, (d) W75, and (e) W100. | ||
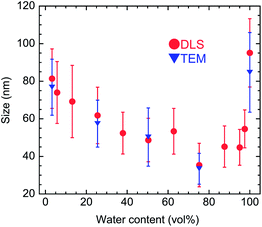 | ||
| Fig. 5 Changes with solvent water content in mean hydrodynamic size (●) and mean size measured from TEM images (▼). Error bars show standard deviations. | ||
3.4 Photoluminescence properties
Fig. 6 shows the PL and PLE spectra of a powdered sample of W75. The PLE spectrum consists of two excitation bands, at ∼238 and ∼360 nm, corresponding to the 4f8 → 4f75d1 transition of Tb3+ and the 4f1 → 5d1 transition of Ce3+, respectively.23,25 The PL spectrum consists of four emission peaks, at 489, 544, 584, and 625 nm, corresponding to the 5D4 → 7F6, 5D4 → 7F5, 5D4 → 7F4, and 5D4 → 7F3 transitions, respectively; these are intrinsic 4f1 → 4f1 transitions of Tb3+.19,23 In contrast, Ce3+-monodoped YBO3 (see Fig. S4 in the ESI†) shows two broad blue emission peaks, at 387 and 415 nm, corresponding to the 5d1 → 4f1(2F5/2) and 5d1 → 4f1(2F7/2) transitions, respectively, of Ce3+. These blue emissions of Ce3+ almost disappear in the PL spectrum of YBO3:Ce3+,Tb3+. Moreover, the excitation spectrum of Tb3+-monodoped YBO3 (see Fig. S5 in the ESI†) has no excitation band in the near-UV region where the 4f1 → 5d1 transitions of Ce3+ are located. These results show that efficient non-radiative energy transfer from Ce3+ to Tb3+ occurs in the YBO3:Ce3+,Tb3+ nanocrystals.The lifetime of the 5D4 → 7F5 emission of Tb3+ is estimated to be 1.81 ms from the decay curve for sample W75 (see Fig. S6 in the ESI†). This is shorter than the lifetime for solid-state-prepared YBO3:Tb3+, ∼4 ms.48 The quantum efficiency for this emission is 0.5%, which is lower than that of solid-state-prepared YBO3:Ce3+,Tb3+.26 The short decay time and low quantum efficiency could be attributed to small nanoparticles with a large number of surface quenching sites. Further work will be done to investigate the predominant factors determining the PL efficiency.
4 Conclusions
We prepared YBO3:Ce3+,Tb3+ nanocrystals by a solvothermal method using a 1,4-butanediol–water mixed solvent, and investigated the influence of the water content of the solvent. A small amount of water significantly promoted crystallization of YBO3:Ce3+,Tb3+. The primary nanocrystal size increased from ∼5 to ∼40 nm with increasing water content of the mixed solvent. The primary nanocrystals prepared at a water content of 75 vol% were well dispersed in the mixed solvent, but the other samples formed secondary assembled particles. These results indicate that not only the size of the primary nanocrystals, but also their assembly can be controlled by changing the composition of the mixed solvent. The well-dispersed nanocrystals with a hydrodynamic size of ∼35 nm showed high transparency to the naked eye and green emission under near-UV irradiation. These nanocrystals could be used in wavelength-converting devices that require high transparency in the visible region, e.g., spectral downshifters for solar cells.Acknowledgements
We thank Tomohiro Sawayama and Seiji Niikura at Sinloihi Co., Ltd. for helpful advice and discussion.References
- D. A. Steigerwald, J. C. Bhat, D. Collins, R. M. Fletcher, M. O. Holcomb, M. J. Ludowise, P. S. Martin and S. L. Rudaz, IEEE J. Sel. Top. Quantum Electron., 2002, 8, 310–320 CrossRef CAS.
- H. A. Höppe, Angew. Chem., Int. Ed., 2009, 48, 3572–3582 CrossRef PubMed.
- S. Ye, F. Xiao, Y. X. Pan, Y. Y. Ma and Q. Y. Zhang, Mater. Sci. Eng., R, 2010, 71, 1–34 CrossRef PubMed.
- W.-Y. Huang, F. Yoshimura, K. Ueda, Y. Shimomura, H.-S. Sheu, T.-S. Chan, H. F. Greer, W. Zhou, S.-F. Hu, R.-S. Liu and J. P. Attfield, Angew. Chem., Int. Ed., 2013, 52, 8102–8106 CrossRef CAS PubMed.
- S. Zhang, IEEE Trans. Plasma Sci., 2006, 34, 294–304 CrossRef CAS.
- M. Bruchez, Jr., M. Moronne, P. Gin, S. Weiss and A. P. Alvisatos, Science, 1998, 281, 2013–2016 CrossRef.
- W. C. W. Chan and S. Nie, Science, 1998, 281, 2016–2018 CrossRef CAS.
- I. L. Medintz, H. T. Uyada, E. R. Goldman and H. Mattoussi, Nat. Mater., 2005, 4, 435–446 CrossRef CAS PubMed.
- H. Dong, L.-D. Sun and C.-H. Yan, Nanoscale, 2013, 5, 5703–5714 RSC.
- H. A. Höppe, M. Daub and M. C. Bröhmer, Chem. Mater., 2007, 19, 6358–6362 CrossRef.
- Z. Xia and R.-S. Liu, J. Phys. Chem. C, 2012, 116, 15604–15609 CAS.
- J. McKittrick, M. E. Hannah, A. Piquette, J. K. Han, J. I. Choi, M. Anc, M. Galvez, H. Lugauer, J. B. Talbot and K. C. Mishra, ECS J. Solid State Sci. Technol., 2013, 2, R3119–R3131 CrossRef CAS PubMed.
- E. Klampaftis, D. Ross, K. R. McIntosh and B. S. Richards, Sol. Energy Mater. Sol. Cells, 2009, 93, 1182–1194 CrossRef CAS PubMed.
- Y. Iso, S. Takeshita and T. Isobe, J. Electrochem. Soc., 2012, 159, J72–J76 CrossRef CAS PubMed.
- C. F. Bohren and D. R. Huffman, Absorption and Scattering of Light by Small Particles, Wiley, New York, 1983, p. 132 Search PubMed.
- M. Saito, Bull. Jpn. Soc. Print. Sci. Technol., 1999, 36, 50–55 CAS.
- S.-S. Liu, D.-K. Ma, Y.-Q. Zhang, P. Cai, X.-A. Chen and S.-M. Huang, CrystEngComm, 2012, 14, 2899–2905 RSC.
- D. Zou, Y. Q. Ma, S. B. Qian, G. H. Zheng, Z. X. Dai, G. Li and M. Z. Wu, J. Alloys Compd., 2013, 574, 142–148 CrossRef CAS PubMed.
- C.-Y. Chung, C.-H. Hsu and C.-H. Lu, J. Am. Ceram. Soc., 2011, 94, 2884–2889 CrossRef CAS.
- K. Olsen, A. Lawler and A. L. Diaz, J. Phys. Chem. C, 2011, 115, 17136–17146 CAS.
- G. Jia, P. A. Tanner, C.-K. Duan and J. Dexpert-Ghys, J. Phys. Chem. C, 2010, 114, 2769–2775 CAS.
- K. Park, J. Kim and K. Y. Kim, Mater. Chem. Phys., 2012, 136, 264–267 CrossRef CAS PubMed.
- H. Ogata, S. Takeshita, T. Isobe, T. Sawayama and S. Niikura, Opt. Mater., 2011, 33, 1820–1824 CrossRef CAS PubMed.
- M. J. Knitel, P. Dorenbos, C. W. E. van Eijk, B. Plasteig, B. Viana, A. Kahn-Harari and D. Vivien, Nucl. Instrum. Methods Phys. Res., Sect. A, 2000, 443, 364–374 CrossRef CAS.
- J.-R. Syu, S. Kumar, S. Das and C.-H. Lu, J. Am. Ceram. Soc., 2012, 95, 1814–1817 CrossRef CAS.
- R. Sato, S. Takeshita, T. Isobe, T. Sawayama and S. Niikura, ESC J. Solid State Sci. Technol., 2012, 1, R163–R168 CrossRef CAS PubMed.
- J. Lin, D. Sheptyakov, Y. Wang and P. Allenspach, Chem. Mater., 2004, 16, 2418–2424 CrossRef CAS.
- L. Guerbous, M. Seraiche and O. Krachni, J. Lumin., 2013, 134, 165–173 CrossRef CAS PubMed.
- L. Chen, A.-Q. Luo, Y. Zhang, X.-H. Chen, H. Liu, Y. Jiang, S.-F. Chen, K.-J. Chen, H.-C. Kuo, Y. Tao and G.-B. Zhang, J. Solid State Chem., 2013, 201, 229–236 CrossRef CAS PubMed.
- D. Boyer, G. Bertrand-Chadeyron, R. Mahiou, C. Caperaa and J.-C. Cousseins, J. Mater. Chem., 1999, 9, 211–214 RSC.
- K. N. Kim, H.-K. Jung and H. D. Park, J. Mater. Res., 2002, 17, 907–910 CrossRef CAS.
- D. Boyer, G. Bertrand-Chaderyon, R. Mahiou, L. Lou, A. Brioude and J. Mugnier, Opt. Mater., 2001, 16, 21–27 CrossRef CAS.
- Z. Wei, L. Sun, C. Liao, J. Yin, X. Jiang and C. Yan, J. Phys. Chem. B, 2002, 106, 10610–10617 CrossRef CAS.
- M. Tukia, J. Hölsa, M. Lastusaari and J. Niittykoski, Opt. Mater., 2005, 27, 1516–1522 CrossRef CAS PubMed.
- D.-S. Kim and R.-Y. Lee, J. Mater. Sci., 2000, 35, 4777–4782 CrossRef CAS.
- S. Hosokawa, Y. Tanaka, S. Iwamoto and M. Inoue, J. Mater. Sci., 2008, 43, 1176–1185 CrossRef.
- X.-C. Jiang, L.-D. Sun, W. Feng and C.-H. Yan, Cryst. Growth Des., 2004, 4, 517–520 CAS.
- X.-C. Jiang, C.-H. Yan, L.-D. Sun, Z.-G. Wei and C.-S. Liao, J. Solid State Chem., 2003, 175, 245–251 CrossRef CAS.
- X.-C. Jiang, L.-D. Sun and C.-H. Yan, J. Phys. Chem. B, 2004, 108, 3387–3390 CrossRef CAS.
- J. Zhang and J. Lin, J. Cryst. Growth, 2004, 271, 207–215 CrossRef CAS PubMed.
- S. Choi, B.-Y. Park and H.-K. Jung, J. Lumin., 2011, 131, 1460–1464 CrossRef CAS PubMed.
- Z. Li, J. Zeng and Y. Li, Small, 2007, 3, 438–443 CrossRef CAS PubMed.
- H. Hara, S. Takeshita, T. Isobe, Y. Nanai, T. Okuno, T. Sawayama and S. Niikura, J. Alloys Compd., 2013, 577, 320–326 CrossRef CAS PubMed.
- A. Tani, H. Hara, S. Takashita and T. Isobe, CrystEngComm, 2013, 15, 10059–10067 RSC.
- Y.-J. Jung, D.-Y. Lim, J.-S. Nho, S.-B. Cho, R. E. Riman and B. W. Lee, J. Cryst. Growth, 2005, 274, 638–652 CrossRef CAS PubMed.
- S. Takeshita, J. Honda, T. Isobe, T. Sawayama and S. Niikura, Cryst. Growth Des., 2010, 10, 4494–4500 CAS.
- L. Zhou and P. O'Brien, Small, 2008, 4, 1566–1574 CrossRef CAS PubMed.
- K.-S. Sohn, Y. Y. Choi and H. D. Park, J. Electrochem. Soc., 2000, 147, 1988–1992 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Fourier-transform infrared spectra, thermogravimetric and differential thermal analysis profiles, Brunauer–Emmett–Teller specific surface areas, photoluminescence decay curves of YBO3:Ce3+,Tb3+ samples, photoluminescence spectra of YBO3:Ce3+ and YBO3:Tb3+ samples. See DOI: 10.1039/c3ra47864e |
| This journal is © The Royal Society of Chemistry 2014 |

