Development of novel alginate lyase cross-linked aggregates for the oral treatment of cystic fibrosis†
Abstract
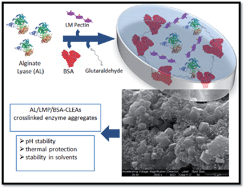
Alginate lyase (AL) from Sphingobacterium multivorum is unsuitable for oral delivery because of its rapid inactivation under acidic conditions. The synthesis of a novel crosslinking enzyme aggregate (CLEA) of AL (AL-CLEA) is proposed. AL precipitation with 95% ammonium sulfate and combined with low methoxylated pectin (LMP), showed 100% precipitation yield. Crosslinking with glutaraldehyde reduced the AL-CLEA activity to less than 1%, but addition of bovine serum albumin (BSA) and LMP during AL-CLEA synthesis increased the activity yield to 14.7%. AL-CLEA exposed to simulated gastric conditions (pH 1.2 to 3.0) showed more than 70% retention of enzymatic activity. Moreover, AL-CLEA showed thermal stability at temperatures over 37 °C. Stability against chemical denaturants (ethanol, acetone and propylene glycol) showed that AL-CLEA was 14 times more stable than free AL in all cases. Finally, a 25% viscosity reduction of alginate solution was achieved with AL-CLEA. This is the first report of AL-CLEA synthesis and evaluation.

 Please wait while we load your content...
Please wait while we load your content...