A freestanding composite film electrode stacked from hierarchical electrospun SnO2 nanorods and graphene sheets for reversible lithium storage†
Abstract
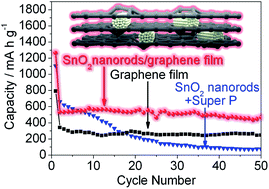
A freestanding hierarchical SnO2 nanorod/graphene composite film electrode was designed and fabricated by a general route involving electrospinning and film casting processes. With dual adaptable strategies (hierarchical nanorod structure and graphene “overcoats”), the composite film electrode exhibited enhanced cycling stability.

 Please wait while we load your content...
Please wait while we load your content...