Rational synthesis of novel π-conjugated poly(1,5-diaminoanthraquinone) for high-performance supercapacitors†
Abstract
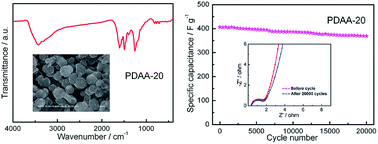
Novel π-conjugated poly(1,5-diaminoanthraquinone) (PDAA) is successfully synthesized using Ce(SO4)2 with suitable redox potential through controlling polymerization temperature. The as-prepared PDAA under optimal conditions displays homogeneous submicron particles, excellent π-conjugated structure as well as high conductivity (1.15 × 10−3 S cm−1). Such unique features make the PDAA an ideal electrode material for electrochemical energy storage. As a supercapacitor electrode, the PDAA exhibits a high specific capacitance (406.3 F g−1), desirable rate performance as well as superior cycle life (9.3% capacitance loss after 20 000 cycles). This simple and cost-effective method without any external additives and stabilizers may provide valuable guidance for the rational preparation of other novel micro-nanostructure π-conjugated conducting polymers.

 Please wait while we load your content...
Please wait while we load your content...