Functional semiconductor–ionic composite GDC–KZnAl/LiNiCuZnOx for single-component fuel cell
Bin Zhu*ab,
Liangdong Fanab,
Yufeng Zhaoc,
Wenyi Tand,
Dingbang Xionge and
Hao Wang*a
aHubei Collaborative Innovation Center for Advanced Organic Chemical Materials, Faculty of Physics and Electronic Technology, Hubei University, Wuhan, Hubei 430062, P.R. China. E-mail: zhubin@hubu.edu.cn; nanoguy@163.com
bDepartment of Energy Technology, Royal Institute of Technology (KTH), S-100 44 Stockholm, Sweden
cKey Laboratory of Applied Chemistry, Department of Environmental and Chemical Engineering, Yanshan University, Qinhuangdao, Hebei 066004, P.R. China
dDepartment of Environment Engineering, Nanjing Institute of Technology, Nanjing 211167, Jiangsu, P.R. China
eNational Lab. for Composite Metallurgy, School of Materials Science and Engineering, Shanghai Jiaotong University, 200240 Shanghai, P.R. China
First published on 27th January 2014
Abstract
The research activities on single-component fuel cells (SCFCs) have opened new doors for keeping ahead with two major areas of focus: improvement of SCFC performances by contributing new materials, and scientific understanding of the SCFC nature and operation mode. The present work reports the exploitation of new material composed of the Gd doped ceria-KAlZn-oxide (GDC–KAZ) and the LiNiCuZn-oxide (LNCZ), combining ionic and semiconducting properties for SCFCs. A new method is first used through an internal electron–hole redox cycle resulting in no net electrons to avoid ceria electronic conduction problems thus to develop an excellent GDC–KAZ electrolyte. Its ionic conductivity, 0.08 S cm−1 at 600 °C, is ten times higher than that of GDC. The SCFC using the GDC–KAZ–LNCZ materials exhibits a remarkable electrochemical performance of 628 mW cm−2 at 580 °C, significantly higher than that of conventional three-component (anode/electrolyte/cathode) fuel cells. The results bring about a new cost-effective and robust system with significant scientific and economic consequences for the fuel cell field.
1. Introduction
Energy conversion technologies with high efficiency and low cost have been in urgent demand as the current energy system does not fulfill the requirements properly. Fuel cells (FCs) have been proved to be very promising for efficient and clean energy conversion. They, on the one hand, will have a significant impact on the global energy system and climate challenge, and on the other hand, still suffer from high investment cost and durability problems due to the inherited complex cell structure, and stern materials and fabrication requirements. Solid oxide fuel cells (SOFCs) as some of the most potential fuel cells, exhibit the most efficient conversion from fuels to electricity, and present the solution for direct utilization of current fossil fuels or renewable bio-fuel.1–3 Global and extensive research activities and enormous technical improvements4–14 have been made since the 60th year of the last century because of increased consumption of fossil fuels and environmental concerns. However, the marketing barrier has not yet been overcome and there is still long way to go before wide implementation. Thus new functional materials and novel system design are urgently desired and expected to speed up the SOFC commercialization.In recent years, nanocomposites for advanced fuel cells (NANOCOFCs)15–17 have caused an emerging R & D upsurge worldwide.17–29 This nanocomposite approach is rationally designed and hence seen in utilizing of the phase interface as a highway for ionic conduction, as well as in the realizing of multi-functionality. Originated from this strategy, advanced ionically conductive composite materials, e.g., core–shell30,31 and nanowire structural32 ceria-based nanocomposite possessing exceptional ionic conductivity (over 0.1 S cm−1 above 300 °C) and a unique simultaneous H+/O2− conduction property,19,33,34 were developed as excellent electrolyte materials for low temperature, 300–600 °C (LT) SOFCs. The promising properties of these nanocomposites also endow them potential application in environmental protection, such as CO2 separation.
More recently, a major breakthrough in this field has been reported, i.e., single-component or electrolyte-free fuel cell (SCFC or EFFC).17,35,36 The SCFC employed only a single component with neither “macroscopic” electrolyte membrane, nor anode and cathode constructions, while presented similar even higher electrochemical performance than the conventional anode/electrolyte/cathode three-component fuel cells. The “three in one”37 simple structure and cost-effectiveness exhibits a great potential to accelerate the FC commercialization.
Since the discovery of SCFC in late of 2010, many fundamental studies have been performed both from experimental and theoretical aspects. Two major research activities are being carried out in parallel. One is designing and developing new materials based on NANOCOFC approach with the aim of improving electrochemical performance of SCFC device; the other is to dig the exact nature and work principle of SCFC. The novel energy conversion technology combines physical and electrochemical principles and analogous p–n junction theory, including solar cell,38 nano-redox process and nano fuel cell.39 Simultaneously, a balance of ion and electron–hole conductivity is very important to achieve higher SCFC electrochemical performance.40 In present work, we therefore develop new functional SCFC materials and performances by combining the newly developed ionic conductor; Gd doped ceria-KAlZn-oxide (GDC–KAZ), and semiconducting LiNiCuZn-oxide (LNCZ), in which the LNCZO not only forms the p–n junction under the applied gas atmospheres for charge separation, but also serves as the dual catalytic function for oxygen reduction and hydrogen oxidation reactions, as demonstrated in previous study.35,36,41 The GDC–KAZ composite was demonstrated to present significantly higher ionic conductivity while much reduced ionic activation energy than the single phase GDC materials. The balance of the ionic and electronic conductivity contributes the excellent single component fuel cell performance.
2. Experimental section
The preparation of GDC–KAlZn (KAZ)–LiNiCuZnOx (LNCZ) ionic–semiconductor materials were performed by two steps.Step one: co-precipitation process of the GDC–KAZ-oxides with the GDC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KAZ in 1
KAZ in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio. Ce(NO3)3·6H2O and Gd(NO3)3·6H2O were dissolved in distilled water with a molar ratio of Ce3+
1 molar ratio. Ce(NO3)3·6H2O and Gd(NO3)3·6H2O were dissolved in distilled water with a molar ratio of Ce3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gd3+ = 4
Gd3+ = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to form a 0.5 mol L−1 solution; the Ce–Gd nitrate solution was drop-wise added into 0.5 mol L−1 Na2CO3 solution under vigorous stirring to form a white precipitate at 120 °C until the final pH value of 9.0. A filtration process is used by sufficient distilled water to wash following a drying process (120 °C in oven). In parallel, KAZ-carbonate precursor was also prepared by co-precipitation process using potassium carbonate (K2CO3) as deposit agent. Aluminum nitrate (Al(NO3)3·9H2O) and zinc nitrate (Zn(NO3)2·6H2O) were dissolved in distilled water with a molar ratio of Al3+
1 to form a 0.5 mol L−1 solution; the Ce–Gd nitrate solution was drop-wise added into 0.5 mol L−1 Na2CO3 solution under vigorous stirring to form a white precipitate at 120 °C until the final pH value of 9.0. A filtration process is used by sufficient distilled water to wash following a drying process (120 °C in oven). In parallel, KAZ-carbonate precursor was also prepared by co-precipitation process using potassium carbonate (K2CO3) as deposit agent. Aluminum nitrate (Al(NO3)3·9H2O) and zinc nitrate (Zn(NO3)2·6H2O) were dissolved in distilled water with a molar ratio of Al3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn2+ = 4
Zn2+ = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to form a 0.5 mol L−1 solution; this solution was drop-wise added into 0.5 mol L−1 K2CO3 solution under vigorous stirring to form a white precipitate at room temperature. The double molar amount of the K2CO3 was used, e.g. 0.2 mol K2CO3 against the 0.1 mol Al3+/Zn2+ to form the deposition. The fixed water, 3000 ml in total amount including the co-precipitation solution is used to wash the deposits. By pouring the top 2000 ml water the rest (ca. 1/3 K+ left) was mixed by above prepared GDC-carbonate precursor powders into with strong stirring and heating at 150 °C until dry. The dried GDC–KAZ-carbonate precursor was ground thoroughly in an agate mortar to obtain the GDC–KAZ-precursor powders following a calcination process at 700 °C in air for 4 h resulting in GDC–KAZ-nanocomposite powders.
3 to form a 0.5 mol L−1 solution; this solution was drop-wise added into 0.5 mol L−1 K2CO3 solution under vigorous stirring to form a white precipitate at room temperature. The double molar amount of the K2CO3 was used, e.g. 0.2 mol K2CO3 against the 0.1 mol Al3+/Zn2+ to form the deposition. The fixed water, 3000 ml in total amount including the co-precipitation solution is used to wash the deposits. By pouring the top 2000 ml water the rest (ca. 1/3 K+ left) was mixed by above prepared GDC-carbonate precursor powders into with strong stirring and heating at 150 °C until dry. The dried GDC–KAZ-carbonate precursor was ground thoroughly in an agate mortar to obtain the GDC–KAZ-precursor powders following a calcination process at 700 °C in air for 4 h resulting in GDC–KAZ-nanocomposite powders.
Step two: the preparation of the GDC–KAZ–LNCZ ionic semiconducting component for EFFCs. At first the LNCZ material was prepared by a slurry method. Li2CO3, NiCO3·3Ni(OH)2·4H2O, Cu2(OH)2·CO3 and ZnCO3 were mixed in a weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4
1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4
0.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8. The mixture was dissolved into an appropriate amount of 1 M HNO3 solution with stirring and heating at 150 °C for 1 h to form sol type slurry. The slurry was then sintered at 800 °C for 2 h to obtain the LNCZ oxide. The above as-prepared GDC–KAZ and LNCZ were mixed in various compositions and grounded completely, followed by a pre-heating at 600 °C for 1 hour. The resulting materials were then grounded again to obtain homogenous GDC–KAZ–LNCZ materials for uses.
0.8. The mixture was dissolved into an appropriate amount of 1 M HNO3 solution with stirring and heating at 150 °C for 1 h to form sol type slurry. The slurry was then sintered at 800 °C for 2 h to obtain the LNCZ oxide. The above as-prepared GDC–KAZ and LNCZ were mixed in various compositions and grounded completely, followed by a pre-heating at 600 °C for 1 hour. The resulting materials were then grounded again to obtain homogenous GDC–KAZ–LNCZ materials for uses.
The samples were analyzed using an X-ray diffractometer (XRD: Rigaku MiniFlex II) using Cu Kα radiation in the 2θ range from 10° to 80°. The microstructures were examined with a scanning electron microscope (SEM: JEOL JSM-7001F), and the different compositions were analyzed in the line scan mode with an energy dispersive X-ray spectroscope (EDS).
The material electrical properties were characterized by electrochemical impedance spectrum (EIS) which were performed on an electrochemical workstation (VersaSTAT 4, Princeton Applied Research, USA) over a frequency range from 100 kHz to 0.01 Hz with excitation potentials of 10 mV.
The fuel cell was fabricated by the dry press process using the GDC–KAZ–LNCZ a single-component SCFC in various compositions,36,38 also including the GDC–KAZ electrolyte based three-component device using the NiO/LiNiOx as the anode and cathode for comparison. The fuel cells were compressed uniaxially under a load of 300 MPa with 13 mm in diameter and 0.8 mm in thickness as well as an active area of 0.64 cm2. Silver paste was applied to both sides of the pellet as a current collector. Hydrogen and air were used as the fuel and the oxidant, respectively. The flow rates were in the range of 80–150 ml min−1 at a pressure of 1 atm for both H2 and air. The SCFC device was measured under a variable resistance load, which adjusts the outputs of cell voltage and power. By collecting data of the cell voltage and current under each resistance load, I (current)–V (voltage) or I–P (power) curve can be drawn from the collected data. A computerized instrument (L-43) was used for fuel cell measurements.
3. Results
Fig. 1 shows room temperature XRD pattern of the sintered GDC–KAZ composite sample. Several phases including the cubic fluorite-type structure CeO2 phase (JCPDS 34-0394), hexagonal ZnO structure (JCPDS 05-0664), alumina phase (JCPDS 02-1422) and trace amount of K2O, can be detected. The as-prepared sample is in a composite status. In fact, composite materials, instead of single phase, can be easily achieved between alumina and ceria42,43 and ZnO.44 Apart from the identified peaks, there are still traces of un-identified peaks but they may not play a significant role due to the trace amount. The EDX element analysis result confirms that K, Al, Zn, Sm, Ce and O compositions co-exist, consistent with XRD result. Especially, the high content K is revealed, which may be doped into Al2O3 or ZnO45 when observing the peaks shift of the obtained XRD patterns compared with the standard diffraction signals.The SEM image of GDC–KAZ is given in Fig. 2A. The GDC–KAZ nanocomposite consists of fine particles smaller than 100 nm in average particle size and show faceted and occasionally irregular shape. It is interesting to note that the particles exhibit a homogeneous distribution with well-connected necks, forming a 3D network structure, which is expected to facilitate the superionic conduction. Fig. 2B and C show the morphologies of the as-prepared LNCZ sample and the GDC–KAZ–LNCZ composite, respectively. The LNCZ particles are found to be of ten to hundred nanometer scale, while GDC–KAZ–LNCZ composite are constructed by both nano-scale GDC–KAZ composite and large LNCZ particles with homogenous distribution for both phases. This is a basic requirement to build continuous networks and charge transport paths for both ionic and semiconducting materials.
Fig. 3A shows the electrochemical impedance spectra of various materials at 500 °C in air, including the pure GDC electrolyte. Fig. 3B shows the temperature dependence of the conductivities for various samples corresponding to Arrhenius plots. It seems that all plots are linearly well-fitted. The conductivities follow the order of GDC ≪ GDC–KAZ < GDC–KAZ–LNCZ < LNCZ.
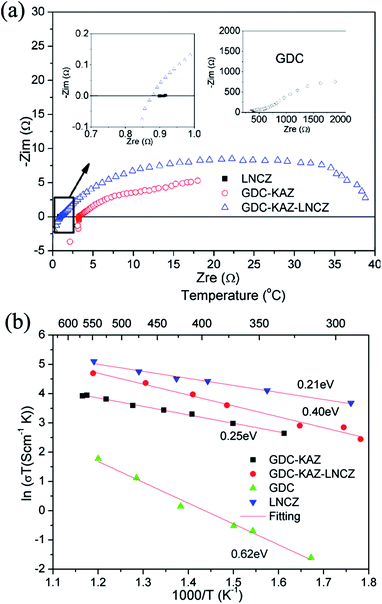 | ||
| Fig. 3 (a) Typical EIS spectra for GDC–KAZ, LNCZ and GDC–KAZ–LNCZ materials in atmosphere at 500 °C and (b) temperature dependence of the conductivities for various samples. | ||
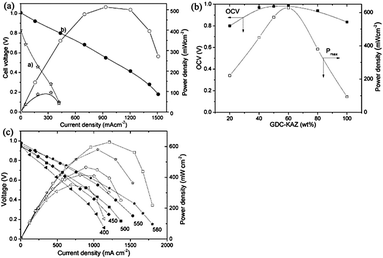
To study the ionic conducting properties of GDC and GDC–KAZ composite, conventional three-component fuel cells were constructed and operated in H2/air condition. Fig. 4A displays the I–V and I–P curves obtained from these two types FCs. This is very clear from the study that the GDC–KAZ electrolyte based FC reaches an OCV of 1.0 V, while the GDC electrolyte FC only gives 0.83 V. Besides, a power density of 510 mW cm−2 is achieved for the GDC–KAZ, but less than 100 mW cm−2 for the GDC case at 580 °C.
The SCFCs were constructed by a single-component of the GDC–KAZ and LNCZ composite with various compositions in a configuration of Ag/GDC–KAZ–LNCZ/Ag, where Ag is served as current collector. The composition adjustment is adopted in order to get an optimized electrochemical performance. Fig. 4B shows the composition dependence of the device OCV (open circuit voltage) and power densities of SCFCs. The device OCVs are observed near 1.0 V in a wide intermediate composition range, containing 40–80 wt% GDC–KAZ (60–20 wt% LNCZ), which is even higher than that for the pure GDC–KAZ case (0.86 V). The best performance, 628 mW cm−2, is achieved at 580 °C at a composition of equal mass ratio of GDC–KAZ and LNZC. The power outputs of SCFC devices show analogous parabola with the gradual increase of the content of GDC–KAZ up to 50 wt% and then decreased afterwards. Both OCVs and current/power density outputs are greatly enhanced in the intermediate composition range between GDC–KAZ and LNCZ. Further analyze the composition dependence of power output, we can see for each individual ion or semiconducting dominant process. For example, when the GDC–KAZ content is less than 20 wt%, the semiconducting material plays major role; when it is above 60 wt%, the ion process dominating the process, the device performances decrease significantly compared to the intermediate region: while when the GDC–KAZ content is in between 25 and 60 wt%, both ion and semiconducting processes function balance together.
Fig. 4C shows typical I–V and I–P characteristics for the best composition SCFC device (50 wt% GDC–KAZ) at various temperatures. The maximum power densities of 320–628 mW cm−2 were achieved between 400 and 580 °C. It should be addressed that, for low temperature e.g. 400–600 °C, the high device performance has more important significance for commercialization.
4. Discussion
The GDC–KAZ is a new system and the material functions are designed based on the following considerations: GDC is classical oxygen ion conductor, while the electrical property of the KAZ-oxide material is somehow complex; the first insight of the material is the mixed electron and hole conduction since K doped ZnO could cause p-conductivity45 and ZnO itself is a n-type conductor;46 the alumina and ceria, form the nanoparticle composite, may have various benefits for the ceria electrolyte materials in enhancing the sintering capability and conductivity.27,29,47 It has been well recognized that ceria reduction produces significant electronic conduction in H2 or FC reduced atmospheres which causes the major problem for such material not successfully to replace YSZ for SOFCs. Extensive efforts have been made to develop the ceria-composite electrolytes by using the 2nd phase materials to overcome the electronic conduction for successful SOFC applications.17,48 Among these materials the core–shell and nanowire doped ceria-based nanocomposites are excellent examples.30,32In this work, we use a different approach to block the electronic conduction, i.e. the extraction method with a p-type semiconducting material. In this method, an optimized potassium dopant to form sufficient p-type K-doped ZnO, which can combine the electrons produced from the ceria material to realize internal electron–hole redox cycle between the ceria and p-conductor, thus guaranteeing no net mobile electrons. In other word, the ion will dominate the electrical properties of the GDC–KAZ which can be used as the electrolyte. The internal electron–hole redox cycle is one of the unique properties for the single component fuel cell.17,35,36 With carefully adjustment of the composition, the single component with mixed ionic and electron–hole conductor can realize the fuel cell function while without internal short circuit. This approach, i.e. electron–hole cycle resulting in no net electron conduction, has been successfully demonstrated in many composites, not only transition metal oxide in our works,35,36 but also the perovskite oxide composite in others.49
This is thus a good scientific approach to develop non-electronic conducting ceria-based electrolyte material. Besides, it can be seen from Fig. 3A that the GDC and GDC–KAZ share the same EIS shapes, a high frequency intercept plus a large tail, which are classical EIS response of ionic conductors. While it should be pointed out that both the real and imaginary impedances, as well as the high frequency intercept of GDC–KAZ composite are much smaller than those of GDC. The single phase GDC EIS typically shows high frequency semicircles for grain (not able to obtain due to the frequency limit) and grain boundary, followed with the electrode effect arc. The resistance value obtained from the high frequency semicircle insertion at Zre axis was used to calculate the material conductivity using cell dimensions. Compared with GDC, the nanocomposite GDC–KAZ materials caused complex and irregular morphologies and interfaces between the constituent individual phases, thus result in a complex EIS.50 In addition to a high frequency grain effect semicircle (not shown due to measured frequency limit), one or two grain boundary semicircle at high frequency range partially overlapping lower frequency semicircles and electrode effect arc. This is probably due to the merged or composite behaviors from grain boundaries which are the results of large amount of the surfaces, contacts and interfaces homogenously distributed among the nano-particles. On the other hand, the LNCZ presents a condensed spot spectrum, see Fig. 3A, this is due to nearly pure electronic conduction behavior without frequency dispersion. While GDC–KAZ–LNCZ exhibits a large depressed semi-arc which is constructed by several semi-arcs due to the combined ionic and electronic conduction nature and the material complex interfaces and microstructures.
From Fig. 3B we can see the nanocomposite GDC–KAZ shows a much lower activation energy, 0.25 eV above 325 °C, compared with the single phase nanometer GDC electrolyte, 0.62 eV, while around 0.9–1.2 eV in micro-particle doped ceria, 0.55–0.8 eV for nanoscale doped ceria.51,52 The highway for interfacial ionic conduction is ascribed to the significantly improved ionic transport in the nanocomposite electrolyte according to theoretical calculation53,54 and experimental observation.55 For example, the “Coulombic model” was used to illustrate the interactions in the interface region and demonstrated the high ionic transport speed in the interface and the extremely low ionic transport activation energy both for oxygen ion and proton conduction.53 An empirical “Swing Model” had been also proposed by XD Wang et al.55 as a possible mechanism of superior proton conduction in the ceria-carbonate composite based on the DC four probe method results. An “effective-medium model” was further suggested to determine the ionic conductivity of the materials core–shelled Samaria doped ceria based composite particles, which does agree well with the experimental data.54 All of these are focused on the interface behavior of the ceria-based composite. It is more reflected from the interface defect, especially for the nano related surface defects and formed space charges. Compared to structural bulk materials, the unique properties of nanostructured material are originally from the large amount of planar defects associating with surface area. In the composite electrolyte, the surface defects of nanoparticles act as ionic highways. The application of the second phase not only preserves a large amount of the surface defects on the ceria oxide, but also helps to suppress the electronic conduction in the fuel cell condition, as demonstrated in the fuel cell testing.
The GDC–KAZ–LNCZ shows a higher ionic activation energy than both LNCZ and GDC–KAZ, which may be attributed to conducting paths block effect due to the composite status of different ionic GDC–KAZ and semiconducting LNCZ phases. Nevertheless, a balance of the ionic and semi-conductivity in SCFC condition is more important issue, resulting in improvement of the material property and electrochemical performance.35,36,40,56
It can be seen from Fig. 4B that an OCV value higher than 1.0 V is obtained for the GDC–KAZ electrolyte based three-component fuel cells, which implies its qualification for an electrolyte; while the pure GDC does exhibit significant electronic leakage resulting in much lower OCV value. Besides, the higher power output from the GDC–KAZ composite electrolyte than that of the GDC is another distinct advantage due to its high ionic conductivity, e.g. 0.08 S cm−1 at 600 °C compared to typical GDC, 0.01 S cm−1 at this temperature.57 The SCFC device performances depend on a strong balance request for ionic and semi-conductivities. Introduction of the ionic composition can make the SCFC device extremely effective for the current and power outputs, and reach peak power output of more than 1200 mA cm−2 and 628 mW cm−2 at 580 °C, see Fig. 4B and C. When the weight content of GDC–KAZ less than 40%, OCVs and current significantly decrease due to unbalanced ions and electrons/holes, a net electronic conduction may cause the device performances decreased. The competing ion and semiconducting conductivities and relevant high performance SCFCs will provide very important knowledge for the material design, optimization and further development.
In our recent work, the SCFC/EFFC operational principles have been proposed39 where nano-redox and nano-fuel cell process on particles have been presented. The main feature of the EFFC was the co-existence of semiconductor and ion conductor forming a bulk hetero-junction (BHJ) structure, which may avoid the internal short circuiting problem, and in the same time realize nano-redox and fuel cell processes at nano-scale, i.e., on that particle level. The operation of the GDC–KAZ–LNCZ SCFC may thus be described corresponding to above suggested nano-redox processes. The detailed experimental determinations on each nano-redox process are sensitive to the composition dependence, particle morphology and size as well as corresponding BHJ structures and internally particles' contacts. All these are deserved for further research and not yet able to be clarified within this work.
The point of the GDC–KAZ–LNCZ material systems developed for advanced SCFCs using the abundant nature source, K, Al, Zn, Li, Ni, Cu oxides, with significant reduced GDC rare-earth material amount can make the material costs much cheaper due to low cost metal oxides and also low amount rare-earth material demand. New advanced fuel cell technology based on this work can be developed rapidly for scalable for commercialization because of the simple design, easy preparation and fabrication technologies, and “three in one” cost-effective productions.
5. Conclusions
The GDC–KAZ and GDC–KAZ–LNCZ composite materials were fabricated as new functional ionic conducting electrolyte and ion-semiconducting single-component materials for FCs and SCFCs, respectively. These materials were designed and developed by combining nano- and composite approach and ion-semiconducting properties. The redox cycle between the n and p materials results no net electrons to realize successful ionic GDC–KAZ electrolyte materials. Based on the ion-semiconducting properties of GDC–KAZ–LNCZ, new functionalities can be developed to meet requirement of high performance SCFC for practical applications. A remarkable fuel cell performance of 628 mW cm−2 was achieved at 580 °C by the optimized composition of GDC–KAZ–LNCZ with balanced ion and semi-conductivities (electron and hole). The results demonstrated here is that, this new material system can be regarded as not only great new scientific field but also a big potential for development of marketable SCFCs products because of high efficiency and easy fabrication from cheaper and adequate nature materials.Acknowledgements
This work is supported in part by the National Nature Science Foundation of China (no. 51372075, 51311130312 and 51202213), Swedish Research Council (VR, no. 621-2011-4983) and EC FP7 TriSOFC project (no. 303454). The lead author would like to thank Hubei provincial 100 talent distinguish professor grant.Notes and references
- N. Q. Minh, J. Am. Ceram. Soc., 1993, 76, 563 CrossRef CAS.
- M. Mogensen, S. H. Jensen, A. Hauch, I. Chorkendorff and T. Jacobsen, Ceram. Eng. Sci. Proc., 2008, 28, 91 CAS.
- L. Malavasi, C. A. J. Fisher and M. S. Islam, Chem. Soc. Rev., 2010, 39, 4370 RSC.
- E. Perry Murray, T. Tsai and S. A. Barnett, Nature, 1999, 400, 649 CrossRef PubMed.
- B. C. H. Steele and A. Heinzel, Nature, 2001, 414, 345 CrossRef CAS PubMed.
- Z. Shao, S. Haile, J. Ahn, P. Ronney, Z. Zhan and S. Barnett, Nature, 2005, 435, 795 CrossRef CAS PubMed.
- L. Yang, S. Z. Wang, K. Blinn, M. F. Liu, Z. Liu and Z. Cheng, et al., Science, 2009, 326, 126 CrossRef CAS PubMed.
- E. Wachsman and K. Lee, Science, 2011, 334, 935 CrossRef CAS PubMed.
- W. Zhou, F. Liang, Z. Shao, J. Chen and Z. Zhu, Sci. Rep., 2011, 1 Search PubMed , article number: 155.
- H. Da, X. Liu, F. Zeng, J. Qian, T. Wu and Z. Zhan, Sci. Rep., 2012, 2 Search PubMed , ariticle number: 462.
- L. Dieterle, P. Bockstaller, D. Gerthsen, J. Hayd, E. Ivers-Tiffée and U. Guntow, Adv. Energy Mater., 2011, 1, 249 CrossRef CAS.
- S. Huang, Q. Lu, S. Feng, G. Li and C. Wang, Adv. Energy Mater., 2011, 1, 1094 CrossRef CAS.
- X.-M. Ge, S.-H. Chan, Q.-L. Liu and Q. Sun, Adv. Energy Mater., 2012, 2, 1156 CrossRef CAS.
- K. Kerman, B. Lai and S. Ramanathan, Adv. Energy Mater., 2012, 2, 656 CrossRef CAS.
- B. Zhu, Int. J. Energy Res., 2009, 33, 1126 CrossRef CAS.
- B. Zhu, J. Nanosci. Nanotechnol., 2011, 11, 8873 CrossRef CAS PubMed.
- B. Zhu, L. Fan and P. Lund, Appl. Energy, 2013, 106, 163 CrossRef CAS PubMed.
- T. Schober, Electrochem. Solid-State Lett., 2005, 8, A199 CrossRef CAS PubMed.
- J. Huang, Z. Mao, Z. Liu and C. Wang, Electrochem. Commun., 2007, 9, 2601 CrossRef CAS PubMed.
- C. Xia, Y. Li, Y. Tian, Q. Liu, Z. Wang and L. Jia, et al., J. Power Sources, 2010, 195, 3149 CrossRef CAS PubMed.
- R. Chockalingam and S. Basu, Int. J. Hydrogen Energy, 2011, 36, 14977 CrossRef CAS PubMed.
- Y. Xia, Y. Bai, X. Wu, D. Zhou, X. Liu and J. Meng, Int. J. Hydrogen Energy, 2011, 36, 6840 CrossRef CAS PubMed.
- A. S. V. Ferreira, C. M. C. Soares, F. M. H. L. R. Figueiredo and F. M. B. Marques, Int. J. Hydrogen Energy, 2011, 36, 3704 CrossRef CAS PubMed.
- L. Zhang, R. Lan, A. Kraft and S. Tao, Electrochem. Commun., 2011, 13, 582 CrossRef CAS PubMed.
- W. Liu, Y. Y. Liu, B. Li, T. D. Sparks, X. Wei and W. Pan, Compos. Sci. Technol., 2010, 70, 181 CrossRef CAS PubMed.
- M. Benamira, A. Ringuedé, V. Albin, R. N. Vannier, L. Hildebrandt and C. Lagergren, et al., J. Power Sources, 2011, 196, 5546 CrossRef CAS PubMed.
- T. Ristoiu, T. Petrisor Jr, M. Gabor, S. Rada, F. Popa and L. Ciontea, et al., J. Alloys Compd., 2012, 532, 109 CrossRef CAS PubMed.
- L. Zhang, N. Xu, X. Li, S. Wang, K. Huang and W. H. Harris, et al., Energy Environ. Sci., 2012, 5, 8310 CAS.
- S. A. Muhammed Ali, A. Muchtar, A. Bakar Sulong, N. Muhamad and E. Herianto Majlan, Ceram. Int., 2013, 39, 5813 CrossRef CAS PubMed.
- X. Wang, Y. Ma, R. Raza, M. Muhammed and B. Zhu, Electrochem. Commun., 2008, 10, 1617 CrossRef CAS PubMed.
- J. Wu, B. Zhu, Y. Mi, S.-J. Shih, J. Wei and Y. Huang, J. Power Sources, 2012, 201, 164 CrossRef CAS PubMed.
- Y. Ma, X. Wang, S. Li, M. S. Toprak, B. Zhu and M. Muhammed, Adv. Mater., 2010, 22, 1640 CrossRef CAS PubMed.
- B. Zhu, I. Albinsson, C. Andersson, K. Borsand, M. Nilsson and B. E. Mellander, Electrochem. Commun., 2006, 8, 495 CrossRef CAS PubMed.
- L. Fan, G. Zhang, M. Chen, C. Wang, J. Di and B. Zhu, Int. J. Electrochem. Sci., 2012, 7, 8420 CAS.
- B. Zhu, R. Raza, H. Qin, Q. Liu and L. Fan, Energy Environ. Sci., 2011, 4, 2986 CAS.
- B. Zhu, R. Raza, G. Abbas and M. Singh, Adv. Funct. Mater., 2011, 21, 2465 CrossRef CAS.
- Nat. Nanotechnol. 2011, 6, 330 Search PubMed, Research Highlight.
- B. Zhu, R. Raza, Q. Liu, H. Qin, Z. Zhu and L. Fan, et al., RSC Adv., 2012, 2, 5066 RSC.
- B. Zhu, P. Lund, R. Raza, J. Patakangas, Q.-A. Huang and L. Fan, et al., Nano Energy, 2013, 2, 1179 CrossRef CAS PubMed.
- Y. Xia, X. Liu, Y. Bai, H. Li, X. Deng and X. Niu, et al., RSC Adv., 2012, 2, 3828 RSC.
- L. Fan, H. Zhang, M. Chen, C. Wang, H. Wang and M. Singh, et al., Int. J. Hydrogen Energy, 2013, 38, 11398 CrossRef CAS PubMed.
- Y. Liu, C. Yin, L. Wang, D. Li, J. Lian and J. Hu, et al., Sci. Sintering, 2008, 40, 13 CrossRef CAS.
- R. Chockalingam, V. R. W. Amarakoon and H. Giesche, J. Eur. Ceram. Soc., 2008, 28, 959 CrossRef CAS PubMed.
- H. Karami, M. A. Karimi and R. Jokar, Synth. React. Inorg., Met.-Org., Nano-Met. Chem., 2011, 41, 603 CrossRef CAS.
- M. K. Gupta, N. Sinha and B. Kumar, J. Appl. Phys., 2011, 109, 083532 CrossRef PubMed.
- J. Anderson and G. V. D. W. Chris, Rep. Prog. Phys., 2009, 72, 126501 CrossRef.
- J.-S. Lee, K.-H. Choi, B.-K. Ryu, B.-C. Shin and I.-S. Kim, Ceram. Int., 2004, 30, 807 CrossRef CAS PubMed.
- L. Fan, C. Wang, M. Chen and B. Zhu, J. Power Sources, 2013, 234, 154 CrossRef CAS PubMed.
- X. Dong, L. Tian, J. Li, Y. Zhao, Y. Tian and Y. Li, J. Power Sources, 2014, 249, 270 CrossRef CAS PubMed.
- Z. Tang, Q. Lin, B.-E. Mellander and B. Zhu, Int. J. Hydrogen Energy, 2010, 35, 2970 CrossRef CAS PubMed.
- J. L. M. Rupp and L. J. Gauckler, Solid State Ionics, 2006, 177, 2513 CrossRef CAS PubMed.
- C. Kleinlogel and L. J. Gauckler, Solid State Ionics, 2000, 135, 567 CrossRef CAS.
- B. Zhu, S. Li and B. E. Mellander, Electrochem. Commun., 2008, 10, 302 CrossRef CAS PubMed.
- Q. Liu and B. Zhu, Appl. Phys. Lett., 2010, 97, 183115 CrossRef PubMed.
- X. Wang, Y. Ma, S. Li, A.-H. Kashyout, B. Zhu and M. Muhammed, J. Power Sources, 2011, 196, 2754 CrossRef CAS PubMed.
- L. Fan, C. Wang, O. Osamudiamen, R. Raza, M. Singh and B. Zhu, J. Power Sources, 2012, 217, 164 CrossRef CAS PubMed.
- T. H. Etsell and S. N. Flengas, Chem. Rev., 1970, 70, 339 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |