Sequential recognition of zinc ion and hydrogen sulfide by a new quinoline derivative with logic gate behavior†
Zhengping Dong*,
Xuanduong Le,
Panpan Zhou,
Chunxu Dong and
Jiantai Ma*
College of Chemistry and Chemical Engineering, Gansu Provincial Engineering Laboratory for Chemical Catalysis, Lanzhou University, Lanzhou 730000, PR China. E-mail: dongzhp@lzu.edu.cn; majiantai@lzu.edu.cn; Fax: +86-0931-8912582; Tel: +86-0931-8912577
First published on 28th February 2014
Abstract
A Schiff base-type fluorescent chemosensor L has been synthesized and characterized to relay recognition of Zn2+ and H2S. Among various metal ions, only Zn2+ induces the fluorescence enhancement of the sensor L and results in an “Off–On” type sensing with excellent selectivity and high sensitivity in aqueous solution. The lowest detection limit for Zn2+ is 1 × 10−7 M. Density functional theory calculation for L and the resultant zinc complex is also performed in this study. The chemosensor also exhibits fluorescence quenching with Cu2+ in aqueous solution. Fluorescent changes of L upon the addition of Zn2+ and Cu2+ is utilized as an INHIBIT logic gate at the molecular level, using Zn2+ and Cu2+ as chemical inputs and the fluorescence intensity signal as the output. On the other hand, the consequent product of L and Zn2+, L–2Zn, is an excellent indicator for H2S for displacement of Zn2+ from the complex L–2Zn. Its H2S sensing behavior is not interfered with by reduced glutathione (GSH), L-cysteine (L-Cys), and even bovine serum albumin (BSA) indicates that L–2Zn is able to detect H2S without any distinct interference from these biological thiols. The addition of H2S leads to the fluorescence quenching of L–2Zn, forming an “On–Off” type sensing system. Therefore, the sensing process for Zn2+ and sequential detection of H2S is a reversible one, and also constitutes an “Off–On–Off” type fluorescence monitoring system. This Zn2+ and H2S sequential recognition via fluorescence relay enhancement and quenching give probe L the potential utility for Zn2+ and H2S detection in aqueous media and biological samples.
Introduction
Fluorescent sensors for the selective detection of various biologically and environmentally relevant metal ions, such as the zinc ion (Zn2+) have recently attracted great attention. Zn2+ is the second most abundant metal ion in the human body, which plays an important role in physiological and metabolic processes.1–3 However, a lack of Zn2+ could result in an increasing risk of different diseases.4,5 Thus the development of new and efficient systems for sensing Zn2+ is of great current interest. Among several chemical tools, fluorescent probes provide the optimal choice for the detection of Zn2+ in biological samples due to their simplicity and high sensitivity. In this regard, a variety of fluorescent Zn2+ probes have been developed based on various fluorophores.6–9 From another viewpoint, a number of fluorescent sensors have been reported as molecular logic gates.10,11 The use of molecular logic gates has become very popular for miniaturization of the information process using ions, photons, pH, temperature or certain molecules as inputs, whereby the observable changes in optical or electrochemical signals are the output data.12On the other hand, organic compounds as sensors for metal ions and resultant metal complexes as sensors for anions or biomolecules have gained popularity recently.13–19 Some of the sequential detection systems work through a cation displacement assay. For instance, the fluorescent sensor binding with the metal ion displays a significant change in its fluorescence intensity. Then the resultant metal ion complex acts as an ideal candidate for the recognition of anions through the liberation of the sensor from the metal complex due to the strong affinity of the anion towards the metal ion; consequently this brings a considerable change in the fluorescence profile of the receptor–metal complex.20
Among most of the essential biomolecules of the human body, hydrogen sulfide (H2S) acts as the third endogenous gaseous signaling compound (gasotransmitter) after NO and CO.21–24 Altered levels of H2S have been linked to many diseases;25,26 for example, at a low concentration, H2S can produce personal distress, whereas at a higher concentration, it can result in loss of consciousness, permanent brain damage, or even death through asphyxiation.27 Therefore, the detection of H2S in living systems has attracted great attention recently.28–30 A few fluorescent sensors for H2S have been reported over the last two years.31–34 However, most of the reported sensors are based on specific H2S-induced reactions and have some drawbacks such as having too long a response time and not being easy to recover. To solve these problems, fluorescent sensors based on cation displacement sensing mechanisms would be the best choice, because the cation displacement process usually occurs within a matter of seconds. The recovered organic fluorophores could coordinate with cations and be reused to recognize H2S. Therefore, it would be highly desirable to employ an organic metal complex as a fluorescence probe for H2S. In this regard, pioneering work has been reported from Nagano's laboratory,35 where they developed a novel fluorescent probe with high selectivity and sensitivity based on azamacrocyclic Cu2+ complex chemistry for H2S.
However, fluorescent sensors that can relay recognition of metal ions and H2S are very rare. Inspired by the importance of sensing of Zn2+ and H2S in physiological samples, the design of fluorescent sensors that can sequentially detect Zn2+ and H2S is very meaningful.
Bearing the above statement in mind, in this work, a fluorescent sensor for Zn2+ and the resultant Zn2+ complex as a sensor for H2S has been designed. Firstly, a quinoline-based Schiff base compound L was synthesized for the detection of Zn2+ since many quinoline-based fluorescent sensors have excellent selectivity for zinc sensing.36,37 Density functional theory calculations for L and the resultant zinc complex are also carried out in this study. The fluorescent changes of L upon the addition of Zn2+ and Cu2+ are utilized as an INHIBIT logic gate at the molecular level. Secondly, the resultant complex L–2Zn was employed as an H2S sensor via the removal of Zn2+ from the complex L–2Zn. As is well known that in the aqueous state under the physiological pH, the major form of H2S exists as HS−, where the ratio of HS−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2S is approximately 3
H2S is approximately 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.38 Thus, in this work, HS− was used as the equivalent of H2S. The L–2Zn ensemble can detect H2S within 2 min, which is much faster than other reported sensors based on H2S-induced reactions, such as azide reduction21,23,39 and nucleophilic reaction to achieve strong fluorescence.22,30 Furthermore, this monitoring process for both Zn2+ and H2S is an “Off–On–Off” monitoring system and could be extended to the design of new receptors for the detection of cations and anions in aqueous solutions.
1.38 Thus, in this work, HS− was used as the equivalent of H2S. The L–2Zn ensemble can detect H2S within 2 min, which is much faster than other reported sensors based on H2S-induced reactions, such as azide reduction21,23,39 and nucleophilic reaction to achieve strong fluorescence.22,30 Furthermore, this monitoring process for both Zn2+ and H2S is an “Off–On–Off” monitoring system and could be extended to the design of new receptors for the detection of cations and anions in aqueous solutions.
Experimental
Reagents and instrumentation
8-Aminoquinoline, glycine, 4-methylphenol, all the cationic compounds and anionic compounds were purchased from Aldrich and used as received. All other chemicals were of the reagent-grade purchased from Tianjing Guangfu Chemical Company and used as supplied. All solvents used for synthesis and measurements were redistilled before use.1H NMR and 13C NMR spectra were obtained using a 400 MHz Varian Unity Inova spectrophotometer. Solid-state 13C CP MAS NMR was carried out using a Bruker Avance IIWB 400 M spectrometer at room temperature. All absorption and emission spectra were recorded at room temperature. UV-Vis absorption spectra were obtained using a Varian UV-Cary100 spectrophotometer. Steady-state luminescence spectra were measured using an Hitachi F-4500 fluorescence spectrophotometer with an excitation wavelength of 360 nm. Quantum yields were determined by an absolute method using an integrating sphere on an Edinburgh Instruments FLS920 spectrometer. All absorption and fluorescence spectra were recorded 2 min after the addition of cations or anions.
UV-Vis and fluorescence titrations
Solutions of compound L were prepared in dry DMF (10−2 M). Metal perchlorates and anions were prepared in H2O. In the titration experiments, each time 2 mL of solution of ligand L (10 μM) were added to a quartz cuvette (path length, 1 cm) and metal ions were added to the quartz cuvette using a micro-pipette. For fluorescence measurements, excitation was provided at 360 nm, and emission was collected from 410 to 630 nm.Synthesis of compound L
The synthesis of chemosensor L was achieved from the condensation of compound 1 and compound 2 in a yield of 70% (Scheme 1, for the detailed experimental sections see ESI†).Results and discussion
The condensation of glycine-N-8-quinolylamide with 2,6-diformyl-4-methylphenol in ethanol provided the quinoline-based compound L in 70% yield (Scheme 1). It is well known that many quinoline-based fluorescent sensors have excellent selectivity for zinc sensing.40–47 Therefore, the synthesized compound L was firstly used as a zinc probe. Subsequently the sequential complex L–2Zn was used as a hydrogen sulfide sensor based on a cation removal sensing mechanism.35Absorption spectroscopy of L and Zn2+
The binding behavior of compound L toward Zn2+ as its chloride salt was examined by UV-Vis spectroscopy. The titration experiment was carried out in CH3CH2OH–Tris–HCl buffer solution (50 mM Tris, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v, pH 7.2) by adding aliquots of Zn2+. The absorption spectrum of compound L (10 μM) was characterized by the presence of a typical absorption band at 320 nm due to the quinoline moiety (Fig. 1). Upon addition of Zn2+ ions (0–3 equiv.), the band at 320 nm was decreased slightly, while a new band at 260 nm increased gradually. This intensity changes of the absorption peaks were likely due to the coordination of L with Zn2+.48 No obvious absorbance enhancement was observed at 260 nm when the concentration of Zn2+ was increased from 2 to 3 equiv., indicating the exclusive formation of a 1
50, v/v, pH 7.2) by adding aliquots of Zn2+. The absorption spectrum of compound L (10 μM) was characterized by the presence of a typical absorption band at 320 nm due to the quinoline moiety (Fig. 1). Upon addition of Zn2+ ions (0–3 equiv.), the band at 320 nm was decreased slightly, while a new band at 260 nm increased gradually. This intensity changes of the absorption peaks were likely due to the coordination of L with Zn2+.48 No obvious absorbance enhancement was observed at 260 nm when the concentration of Zn2+ was increased from 2 to 3 equiv., indicating the exclusive formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex between L and Zn2+ (Fig. 1a inset). A Job plot also confirmed the 1
2 complex between L and Zn2+ (Fig. 1a inset). A Job plot also confirmed the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometric ratio of L with Zn2+ (Fig. 1b).
2 stoichiometric ratio of L with Zn2+ (Fig. 1b).
Fluorescence response of L and Zn2+
The fluorescence spectroscopic study is also an important method to investigate the zinc-binding behavior of the fluorescent probe L. In the fluorescence spectrum, compound L (10 μM) showed a weak emission at 482 nm when it was excited at 360 nm (Fig. 2). The weak fluorescence emission of compound L can be explained by two reasons as described in other reported quinoline-based sensors42,49,50 and Schiff base-type sensors.51 Firstly, the six nitrogen atoms of L can form intramolecular hydrogen bonds with hydrogen atoms in the absence of metal ions, which results in a photo-induced electron-transfer, and the de-excitation of the resulting tautomer occurs mainly through a non-radiative pathway.42,49,50,52 Secondly, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization is also a decay process of excited states in compounds with an unbridged C
N isomerization is also a decay process of excited states in compounds with an unbridged C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N structure so those compounds are often non-fluorescent.51 As a result, compound L emits only weak fluorescence. Upon addition of 2.0 equiv. of Zn2+ ions to the solution of sensor L, the intramolecular hydrogen bond is inhibited, the C
N structure so those compounds are often non-fluorescent.51 As a result, compound L emits only weak fluorescence. Upon addition of 2.0 equiv. of Zn2+ ions to the solution of sensor L, the intramolecular hydrogen bond is inhibited, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization is also inhibited, thus leading to a significant increase in the fluorescence emission. The fluorescence intensity of L increased gradually as a function of Zn2+ ion concentration (Fig. 2 inset). When more than 2.0 equiv. of Zn2+ was added, the fluorescence intensity became constant afterwards. The detection limit of L as a fluorescent sensor for the analysis of Zn2+ was found to be 0.1 μM from the titration experiment. The fluorescence quantum yield of compound L in the free state was found to be 7.9% and for the Zn2+-bound state was found to be 11.33%.
N isomerization is also inhibited, thus leading to a significant increase in the fluorescence emission. The fluorescence intensity of L increased gradually as a function of Zn2+ ion concentration (Fig. 2 inset). When more than 2.0 equiv. of Zn2+ was added, the fluorescence intensity became constant afterwards. The detection limit of L as a fluorescent sensor for the analysis of Zn2+ was found to be 0.1 μM from the titration experiment. The fluorescence quantum yield of compound L in the free state was found to be 7.9% and for the Zn2+-bound state was found to be 11.33%.
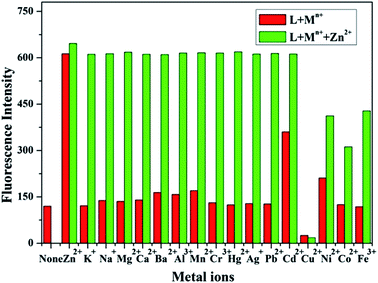
Cation selectivity assays were performed for sensor L with an excitation energy of 360 nm while keeping other experimental conditions unchanged (Fig. 3, red bars). Fig. 3 illustrates the fluorescence intensities of sensor L in the presence of various metal ions under physiological conditions (CH3CH2OH–Tris–HCl buffer solution, pH 7.2). The results showed that, among the metal ions studied, only Zn2+ has a significant effect on the fluorescence intensity of the sensor L. Other cations which exist at high concentrations in living cells, e.g., Ca2+, Mg2+, Na+, and K+, did not enhance the fluorescence intensity, as shown in Fig. 3. These results were presumably due to the poor complexation of alkali metals or alkaline earth metals with sensor L. However, a fluorescence increase of Cd2+ was also observed; this is because Zn2+ and Cd2+ are in the same group of the periodic table and cause similar spectral changes when coordinated with fluorescent sensors.45 However, Cd2+ is rarely present in biological systems and would not cause any problem when using sensor L in biological applications.
The fluorescence intensity of sensor L in the presence of 5 equiv. of Zn2+ mixed with various metal ions (5 equiv.) was measured (Fig. 3, green bars) in cation competition studies. In the presence of most of the investigated cations, the emission intensity of Zn2+-bound L was unperturbed. However, Cu2+, Ni2+, Co2+ and Fe3+ caused fluorescence quenching to some extent, due to the displacement of Zn2+ by these cations.49,53 However, Cu2+, Ni2+ and Co2+ ions would have little influence in vivo, since they exist at very low concentrations and are negligible in normal biological samples. Thus, Zn2+ can be distinguished from these investigated ions under physiological conditions.
In addition to metal ion selectivity, the fluorescence intensity of sensor L at various pH values in the presence and absence of Zn2+ was also measured (Fig. 4). In the absence of Zn2+, protons did not induce any obvious fluorescence enhancement in the range of pH < 6.5, but slight increase was observed at pH > 6.5. The reason was that the formation of the intramolecular hydrogen bond was forbidden in alkaline solution. When Zn2+ was added, sensor L showed no appreciable sensing ability for Zn2+ at pH < 6.5, which could be due to the competition of H+ below pH 6.5. However, sensor L exhibited satisfactory Zn2+ sensing abilities when the pH was increased to the range 6.5–9. At pH = 7.2, the fluorescence intensity reached its maximum value, indicating that sensor L possessed the highest sensing ability in an environment similar to the physiological environment (pH = 7.2).
 | ||
Fig. 4 Fluorescence intensity of L (10 μM) at various pH values in CH3CH2OH–Tris–HCl buffer solution (50 mM Tris, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v) in the absence and presence of Zn2+ (5 equiv.), λex = 360 nm. 50, v/v) in the absence and presence of Zn2+ (5 equiv.), λex = 360 nm. | ||
Logic gates based on L with Zn2+ and Cu2+ as the inputs
It has been reported that fluorescence sensors can act as logic gates.10 Based on the interaction of L with Zn2+ and Cu2+ ions with subsequent changes of its emission intensity, a binary INHIBIT type logic gate by employing Zn2+ and Cu2+ as inputs was constructed. In order to elucidate the design of the logic gate, binary “0” and binary “1” are assigned to the inputs and outputs. For the input signals, binary “0” stands for no addition of Zn2+/Cu2+. Binary “1” denotes addition of Zn2+/Cu2+. The output values (fluorescence intensity) below a pre-defined threshold level are translated into binary “0”, while the fluorescence output values above the threshold correspond to binary “1”, in accordance with positive logic convention. The four possible input combinations are (0,0), (1,0), (0,1) and (1,1) shown in Fig. 5. Free L shows a weak fluorescence emission at 482 nm (“0”). In the presence of Zn2+ ion the fluorescence is strong (“1”). In the presence of Cu2+ ion the fluorescence is low (“0”). In the simultaneous presence of both Zn2+ and Cu2+ ions, the system still exhibits a low fluorescence (“0”). Therefore, a two-input INHIBIT logic gate was successfully mimicked for L. | ||
| Fig. 5 The combinatorial logic scheme (top) of INHIBIT logic operations and the truth table (bottom). | ||
Quantum mechanical calculations
Moreover, quantum mechanical calculations were carried out to confirm the configuration of L–2Zn, in which the B3LYP54,55 function was used. For atoms C, H, N, Cl and O in this compound, the 6-31G(d,p) basis set was used, whereas the LANL2DZ basis set was used for the Zn atom. The calculations were performed using the Gaussian 09 suite of programs.56 The optimized configuration of L and L–2Zn complex are depicted in Fig. 6a and 6b. The results show that there are two Zn2+ ions binding to L via five coordination sites, and the two Zn2+ ions coordinate with the O atom of para-methyl-substituted phenol and one Cl atom simultaneously. The Zn–O bond length is 2.090 Å (Zn–Ophenol–oxygen), the Zn–Cl bond length is 2.457 Å, and the Zn–N bond lengths are 2.058 Å (Zn–Namino–nitrogen), 2.182 Å (Zn–Nquinoline–nitrogen) and 2.206 Å (Zn–NSchiff base–nitrogen). It can be concluded that L can provide suitable space to accommodate the Zn2+ and Cl− ions, forming a stable compound. | ||
| Fig. 6 The optimized configuration of (a) L, (b) L with Zn2+. HOMO–LUMO energy gaps for respective compounds and interfacial plots of the orbitals: (c) free L and (d) L–2Zn. | ||
Additionally, the electronic properties of L and the L–2Zn complex were also analyzed. It has been shown that the energy gap between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) as well as the electronic distributions may be used to explain the changes in the fluorescent properties upon metal cation coordination and therefore detect the ratiometric response to metal cations.57,58 With respect to L, the HOMO is mainly located on one of its aminoquinoline units, whereas the LUMO is mainly located on the phenol unit (Fig. 6c). But for the binding of two Zn2+ and one Cl− ions to L, the HOMO is mainly located on the two aminoquinoline units, whereas the LUMO is mainly located on the phenol unit (Fig. 6d). Evidently, the binding of two Zn2+ and one Cl− ions to L forces L to redistribute its electron density to stabilize the resulting L–2Zn complex formed. Compared to L, the energy level of the HOMO ofthe L–2Zn complex increases, but the LUMO decreases, so the HOMO–LUMO energy gaps for the L–2Zn complex are smaller than L. These changes can be ascribed to the electron redistribution after the binding. Thereby, it is indicated that the enhanced fluorescence spectra upon the binding of two Zn2+ and one Cl− ions to L arises from the energy level changes caused by the electron redistribution.
Fluorescence spectroscopy of L–2Zn and H2S
Recovery and reuse are important properties for a chemosensor toward the analyte.59–61 In this study, considering the fact that Zn2+ can coordinate with sulfide anions to form a highly stable species ZnS, which has an extremely small solubility product constant (1.2 × 10−23), the obtained fluorescence on the L–2Zn system is regarded as a promising ensemble for the fluorescent “On–Off” detection of H2S via the Zn2+ displacement approach. Also in the H2S selectivity experiment, it is very exciting and noteworthy that compound L can be regenerated only by adding HS− to the solution containing L–2Zn. Thus, a sequential detection system is constructed, not only for Zn2+ detection, but also for the sensing of H2S, as HS− is equivalent to H2S in physiological solution.38In addition, competition experiments were also carried out to explore the anti-interference ability of L–2Zn. Complex L–2Zn (10 μM) was treated with 10 equiv. of various anions, including AcO−, Br−, Cl−, I−, CN−, NO3−, NO2−, SO32−, PO43− and SO42−. As shown in Fig. 7 (black bars), only HS− caused considerable fluorescence quenching, whereas PO43− brought about a slight decrease in the fluorescence intensity. In the anion competition experiments, the L–2Zn ensemble (10 μM) was treated with HS− (10 equiv.) in the presence of various tested anions (10 equiv.) in pH 7.2 CH3CH2OH–Tris–HCl (50 mM Tris, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v) buffer. Also, the results showed that all of the relevant anions tested have virtually no influence on the fluorescence detection of HS− (Fig. 7, red bars). In addition, some thiol-containing amino acids such as reduced glutathione (GSH), L-cysteine (L-Cys) and even bovine serum albumin (BSA) were also examined to further evaluate the H2S selectivity. As with other reported organic metal complex fluorescent probes for H2S,35 the addition of these thiol-containing amino acids to L–2Zn did not induce any noticeable fluorescence intensity changes. Thus, the complex L–2Zn has a very high selectivity for H2S.
50, v/v) buffer. Also, the results showed that all of the relevant anions tested have virtually no influence on the fluorescence detection of HS− (Fig. 7, red bars). In addition, some thiol-containing amino acids such as reduced glutathione (GSH), L-cysteine (L-Cys) and even bovine serum albumin (BSA) were also examined to further evaluate the H2S selectivity. As with other reported organic metal complex fluorescent probes for H2S,35 the addition of these thiol-containing amino acids to L–2Zn did not induce any noticeable fluorescence intensity changes. Thus, the complex L–2Zn has a very high selectivity for H2S.
The mechanism of the sensing of H2S can be described in Scheme 2. When Zn2+ is added, the intramolecular hydrogen bond of compound L is broken, and the lone pair of electrons on the nitrogen atoms is involved in coordination with the Zn2+, leading to a significant increase in the fluorescence emission. And the fluorescence is recovered by the addition of H2S to L–2Zn ensemble, which is attributed to the low solubility product constant of ZnS. With alternately adding Zn2+ and H2S, compound L shows an “Off–On–Off” type fluorescence change, meaning that the detection process is a reversible one for sequential sensing.62
To further understand the sensing behavior of L–2Zn for H2S, an H2S titration experiment was conducted. The fluorescence titration experiments of L–2Zn with H2S were recorded in pH 7.2 CH3CH2OH–Tris–HCl (50 mM Tris, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v) buffer solution. As can be seen in Fig. 8, in the absence of H2S, the free L–2Zn displayed quite a high fluorescence. Importantly, with the addition of NaHS (0–10 equiv.), the fluorescence intensity of L–2Zn decreased gradually at 482 nm due to the displacement of Zn2+ from the complex L–2Zn. The detection limit of H2S could reach the 10−6 M level from the titration experiment.
50, v/v) buffer solution. As can be seen in Fig. 8, in the absence of H2S, the free L–2Zn displayed quite a high fluorescence. Importantly, with the addition of NaHS (0–10 equiv.), the fluorescence intensity of L–2Zn decreased gradually at 482 nm due to the displacement of Zn2+ from the complex L–2Zn. The detection limit of H2S could reach the 10−6 M level from the titration experiment.
Conclusion
In conclusion, we have synthesized a multi-responsive and selective sensor L as a probe to monitor Zn2+ and for the sequential detection of H2S. The receptor showed high sensitivity and selectivity for Zn2+ detection with a detection limit of 10−7 M. And the density functional theory calculation of sensor L and L–Zn was further studied: the results obtained here were very consistent with the experimental results. The fluorescent intensity of compound L can be recovered by adding HS− to the Zn2+ sensing system via the Zn2+ displacement approach. Therefore, the Zn2+ complex can be used for H2S recognition. With alternately adding Zn2+ and H2S, compound L shows an “Off–On–Off” type fluorescence change, meaning that the detection process is a reversible one. And the cation displacement approach employing a metal-complex for anion recognition allows for the design new receptors for the sensitive detection of anions in aqueous solutions.Acknowledgements
The authors acknowledge financial support from the NSFC (Grants 21301082), the Natural Science Foundation of Gansu (no. 1308RJYA028) and the Fundamental Research Funds for the Central Universities (lzujbky-2013-61). The technical support from the Super Computing Center of Gansu Province is also acknowledged.Notes and references
- S. L. Sensi, D. Ton-That, J. H. Weiss, A. Rothe and K. R. Gee, Cell Calcium, 2003, 34, 281–284 CrossRef CAS.
- C. J. Frederickson and A. I. Bush, BioMetals, 2001, 14, 353–366 CrossRef CAS.
- L. A. Finney and T. V. O'Halloran, Science, 2003, 300, 931–936 CrossRef CAS.
- F. Qian, C. L. Zhang, Y. M. Zhang, W. J. He, X. Gao, P. Hu and Z. J. Guo, J. Am. Chem. Soc., 2009, 131, 1460–1468 CrossRef CAS.
- A. K. Singh, S. Mehtab, U. P. Singh and V. Aggarwal, Anal. Bioanal. Chem., 2007, 388, 1867–1876 CrossRef CAS.
- J. L. Vinkenborg, S. M. J. van Duijnhoven and M. Merkx, Chem. Commun., 2011, 47, 11879–11881 RSC.
- M. A. Namdarghanbari, J. Meeusen, G. Bachowski, N. Giebel, J. Johnson and D. H. Petering, J. Inorg. Biochem., 2010, 104, 224–231 CrossRef CAS.
- Y. Mikata, Y. Sato, S. Takeuchi, Y. Kuroda, H. Konno and S. Iwatsuki, Dalton Trans., 2013, 42, 9688–9698 RSC.
- J. E. Kwon, S. Lee, Y. You, K.-H. Baek, K. Ohkubo, J. Cho, S. Fukuzumi, I. Shin, S. Y. Park and W. Nam, Inorg. Chem., 2012, 51, 8760–8774 CrossRef CAS.
- K. Szacilowski, Chem. Rev., 2008, 108, 3481–3548 CrossRef CAS.
- A. P. de Silva, Chem. – Asian J., 2011, 6, 750–766 CrossRef.
- A. P. de Silva and N. D. McClenaghan, Chem. – Eur. J., 2004, 10, 574–586 CrossRef.
- X. Cao, W. Lin and L. He, Org. Lett., 2011, 13, 4716–4719 CrossRef CAS.
- L. Tang, P. Zhou, K. Zhong and S. Hou, Sens. Actuators, B, 2013, 182, 439–445 CrossRef CAS.
- L. Tang, N. Wang, Q. Zhang, J. Guo and R. Nandhakumar, Tetrahedron Lett., 2013, 54, 536–540 CrossRef CAS.
- H. Wang, L. Xue and H. Jiang, Org. Lett., 2011, 13, 3844–3847 CrossRef CAS.
- Q. Zou, X. Li, J. Zhang, J. Zhou, B. Sun and H. Tian, Chem. Commun., 2012, 48, 2095–2097 RSC.
- X. Huang, Z. Guo, W. Zhu, Y. Xie and H. Tian, Chem. Commun., 2008, 5143–5145 RSC.
- W. Zhu, X. Huang, Z. Guo, X. Wu, H. Yu and H. Tian, Chem. Commun., 2012, 48, 1784–1786 RSC.
- K. Kaur, V. K. Bhardwaj, N. Kaur and N. Singh, Inorg. Chim. Acta, 2013, 399, 1–5 CrossRef CAS.
- A. R. Lippert, E. J. New and C. J. Chang, J. Am. Chem. Soc., 2011, 133, 10078–10080 CrossRef CAS.
- C. Liu, J. Pan, S. Li, Y. Zhao, L. Y. Wu, C. E. Berkman, A. R. Whorton and M. Xian, Angew. Chem., Int. Ed., 2011, 50, 10327–10329 CrossRef CAS.
- H. Peng, Y. Cheng, C. Dai, A. L. King, B. L. Predmore, D. J. Lefer and B. Wang, Angew. Chem., Int. Ed., 2011, 50, 9672–9675 CrossRef CAS.
- J. Liu, Y.-Q. Sun, J. Zhang, T. Yang, J. Cao, L. Zhang and W. Guo, Chem. – Eur. J., 2013, 19, 4717–4722 CrossRef CAS.
- T. Liu, X. Zhang, Q. Qiao, C. Zou, L. Feng, J. Cui and Z. Xu, Dyes Pigm., 2013, 99, 537–542 CrossRef CAS.
- Y. C. Chen, C. C. Zhu, Z. H. Yang, J. J. Chen, Y. F. He, Y. Jiao, W. J. He, L. Qiu, J. J. Cen and Z. J. Guo, Angew. Chem., Int. Ed., 2013, 52, 1688–1691 CrossRef CAS.
- P. Patnaik, A Comprehensive Guide to the Hazardous Properties of Chemical Substances, Wiley, New York, 3rd edn, 2007 Search PubMed.
- J. Bae, M. G. Choi, J. Choi and S.-K. Chang, Dyes Pigm., 2013, 99, 748–752 CrossRef CAS.
- W. M. Xuan, C. Q. Sheng, Y. T. Cao, W. H. He and W. Wang, Angew. Chem., Int. Ed., 2012, 51, 2282–2284 CrossRef CAS.
- Y. Qian, L. Zhang, S. T. Ding, X. Deng, C. He, X. E. Zheng, H. L. Zhu and J. Zhao, Chem. Sci., 2012, 3, 2920–2923 RSC.
- B. F. Chen, W. Li, C. Lv, M. M. Zhao, H. W. Jin, H. F. Jin, J. B. Du, L. R. Zhang and X. J. Tang, Analyst, 2013, 138, 946–951 RSC.
- W. Sun, J. L. Fan, C. Hu, J. F. Cao, H. Zhang, X. Q. Xiong, J. Y. Wang, S. Cui, S. G. Sun and X. J. Peng, Chem. Commun., 2013, 49, 3890–3892 RSC.
- K. Zheng, W. Lin and L. Tan, Org. Biomol. Chem., 2012, 10, 9683–9688 CAS.
- Y. Qian, B. Y. Yang, Y. N. Shen, Q. R. Du, L. Lin, J. Lin and H. L. Zhu, Sens. Actuators, B, 2013, 182, 498–503 CrossRef CAS.
- K. Sasakura, K. Hanaoka, N. Shibuya, Y. Mikami, Y. Kimura, T. Komatsu, T. Ueno, T. Terai, H. Kimura and T. Nagano, J. Am. Chem. Soc., 2011, 133, 18003–18005 CrossRef CAS.
- X. M. Meng, S. X. Wang, Y. M. Li, M. Z. Zhu and Q. X. Guo, Chem. Commun., 2012, 48, 4196–4198 RSC.
- M. Mameli, M. C. Aragoni, M. Arca, M. Atzori, A. Bencini, C. Bazzicalupi, A. J. Blake, C. Caltagirone, F. A. Devillanova, A. Garau, M. B. Hursthouse, F. Isaia, V. Lippolis and B. Valtancoli, Inorg. Chem., 2009, 48, 9236–9249 CrossRef CAS.
- F. Hou, J. Cheng, P. Xi, F. Chen, L. Huang, G. Xie, Y. Shi, H. Liu, D. Bai and Z. Zeng, Dalton Trans., 2012, 41, 5799–5804 RSC.
- L. A. Montoya and M. D. Pluth, Chem. Commun., 2012, 48, 4767–4769 RSC.
- D. L. Lu, L. G. Yang, Z. D. Tian, L. Z. Wang and J. L. Zhang, RSC Adv., 2012, 2, 2783–2789 RSC.
- Y. Mikata, A. Yamashita, K. Kawata, H. Konno, S. Itami, K. Yasuda and S. Tamotsu, Dalton Trans., 2011, 40, 4976–4981 RSC.
- G. Xie, P. Xi, X. Wang, X. Zhao, L. Huang, F. Chen, Y. Wu, X. Yao and Z. Zeng, Eur. J. Inorg. Chem., 2011, 2011, 2927–2931 CrossRef CAS.
- Y. L. Cai, X. M. Meng, S. X. Wang, M. Z. Zhu, Z. W. Pan and Q. X. Guo, Tetrahedron Lett., 2013, 54, 1125–1128 CrossRef CAS.
- H. Zhu, J. Fan, J. Lu, M. Hu, J. Cao, J. Wang, H. Li, X. Liu and X. Peng, Talanta, 2012, 93, 55–61 CrossRef CAS.
- X. Zhou, P. Li, Z. Shi, X. Tang, C. Chen and W. Liu, Inorg. Chem., 2012, 51, 9226–9231 CrossRef CAS.
- A. Rajapakse and K. S. Gates, J. Org. Chem., 2012, 77, 3531–3537 CrossRef CAS.
- S. Patra, R. Gunupuru, R. Lo, E. Suresh, B. Ganguly and P. Paul, New J. Chem., 2012, 36, 988–1002 RSC.
- C. J. Gao, X. J. Jin, X. H. Yan, P. An, Y. Zhang, L. L. Liu, H. Tian, W. S. Liu, X. J. Yao and Y. Tang, Sens. Actuators, B, 2013, 176, 775–781 CrossRef CAS.
- X. Y. Zhou, B. R. Yu, Y. L. Guo, X. L. Tang, H. H. Zhang and W. S. Liu, Inorg. Chem., 2010, 49, 4002–4007 CrossRef CAS.
- Y. Zhang, X. Guo, W. Si, L. Jia and X. Qian, Org. Lett., 2008, 10, 473–476 CrossRef CAS PubMed.
- J. Wu, W. Liu, J. Ge, H. Zhang and P. Wang, Chem. Soc. Rev., 2011, 40, 3483–3495 RSC.
- V. Bhalla, Roopa and M. Kumar, Dalton Trans., 2013, 42, 975–980 RSC.
- Y. Mikata, M. Wakamatsu, A. Kawamura, N. Yamanaka, S. Yano, A. Odani, K. Morihiro and S. Tamotsu, Inorg. Chem., 2006, 45, 9262–9268 CrossRef CAS PubMed.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785 CrossRef CAS.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, N. J. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- A. J. Zucchero, P. L. McGrier and U. H. F. Bunz, Acc. Chem. Res., 2009, 43, 397–408 CrossRef PubMed.
- Y.-P. Li, H.-R. Yang, Q. Zhao, W.-C. Song, J. Han and X.-H. Bu, Inorg. Chem., 2012, 51, 9642–9648 CrossRef CAS PubMed.
- X. Tian, Z. P. Dong, R. Wang and J. T. Ma, Sens. Actuators, B, 2013, 183, 446–453 CrossRef CAS.
- Y. Zhang, L. Gao, L. Wen, L. Heng and Y. Song, Phys. Chem. Chem. Phys., 2013, 15, 11943–11949 RSC.
- S. Heng, M.-C. Nguyen, R. Kostecki, T. M. Monro and A. D. Abell, RSC Adv., 2013, 3, 8308–8317 RSC.
- F. Hou, L. Huang, P. Xi, J. Cheng, X. Zhao, G. Xie, Y. Shi, F. Cheng, X. Yao, D. Bai and Z. Zeng, Inorg. Chem., 2012, 51, 2454–2460 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra47755j |
| This journal is © The Royal Society of Chemistry 2014 |