Immobilization of transition metal (Fe2+, Co2+, VO2+ or Cu2+) Schiff base complexes onto graphene oxide as efficient and recyclable catalysts for epoxidation of styrene
Hailiang Sua,
Zhifang Lia,
Qisheng Huob,
Jingqi Guan*a and
Qiubin Kan*a
aCollege of Chemistry, Jilin University, Changchun, 130023, P.R. China. E-mail: guanjq@jlu.edu.cn; qkan@mail.jlu.edu.cn; Tel: +86 431 88499140
bState Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, P.R. China
First published on 31st January 2014
Abstract
Transition metal (Fe2+, Co2+, VO2+ or Cu2+) Schiff base complexes were immobilized onto graphene oxide previously functionalized with 3-aminopropyltriethoxysilane (3-APTES). X-ray diffraction (XRD), IR spectroscopy, thermal gravimetric analyses (TGA) and inductively coupled plasma atomic emission spectroscopy (ICP-AES) confirmed the successful incorporation of the metal Schiff base onto the graphene oxide. N2 adsorption–desorption, scanning electron microscopy (SEM) and transmission electron microscopy (TEM) showed the intact structure of the graphene oxide. Catalytic results showed that the immobilized copper(II) Schiff base complex catalyst was more active than the immobilized iron(II), cobalt(II) and oxovanadium(IV) complexes in the epooxidation of styrene. Above 94% styrene conversion and excellent ∼99% selectivity to the epoxide could be achieved over the copper(II) Schiff base heterogeneous catalyst in the epoxidation of styrene using tert-butyl hydroperoxide (TBHP) as oxidant after 7 h reaction. The recycling experiment results indicated that the catalyst could maintain very high styrene conversion (>93%) and epoxide selectivity (>99%) even after being used for four cycles.
Introduction
The catalytic epoxidation of olefins is an important theme in the production of fine chemicals,1 pharmaceuticals2 and agrochemicals,3 because these products are valuable intermediates for production of chemicals such as polyethers, diots and aminoalcohols in industry. Conventional epoxidation catalysts (i.e. soluble transition metal salts and transition metal complexes) have been extensively used for homogeneous epoxidation of organic substrates such as cyclohexene, cyclooctene and styrene due to their high catalytic performance.4–6However, the application of these catalysts are highly hampered due to the high costs with the problem to remove these catalysts from the reaction system and ambient contaminate. As a consequence, many methods and strategies were explored to immobilize the homogeneous catalysts on some insoluble supports such as organic polymers,7 mesoporous silica,8 zeolite Y,9 zeolite X10 and clays,11 etc. For instance, Visuvamithiran et al. reported that Co(II)–Schiff immobilized on KIT-6 through covalent bond was efficient in the epoxidation of olefins using hydrogen peroxide as oxidant, which could be recycled five cycles without loss of its catalytic activity.12 Kureshy et al. reported Mn(III) salen complexes immobilized onto the layers of the montmorillonite type clay by a simple cation exchange method, which showed high activity and good recoverability in the epoxidation of nonfunctionalized alkenes.13
Recently, metal acetylacetone complexes have been grafted onto supports as heterogeneous catalysts for the oxidation of olefins or other substances. Dorbe et al. reported that oxidovanadium(IV) acetylacetone [VO(acac)2] immobilized onto nanostructure ordered carbon material CMK-3 exhibited good catalytic activity in the epoxidation of geraniol using tert-butyl hydroperoxide as oxidant.14 Esnaashari et al. reported that molybdenyl acetylacetone supported on mult-wall carbon nanotubes were successfully applied in the epoxidation of different alkenes such as cyclic and linear ones.15
In recent years, graphene has been raised great expectation in the field of sensor, drugs deliver, composite materials, catalysis and solar cells due to its unique nanostructure and remarkable properties.16–21 As one of products obtained from graphene, graphene oxide also has its own advantages compared with graphene. For example, graphene oxide is heavily oxygenated and has hydroxyl and epoxide groups on its basal planes as well as carbonyl and carboxyl groups located at the sheet edges.22–25 The plenty oxygenic functional groups in graphene oxide could immobilize various homogeneous materials as active sites. Scheuermann et al. reported that graphene oxide chemically derived from graphene was employed as support for Pd nanoparticles for the Suzuki-Miyaura Coupling Reaction and showed high activity and low palladium leaching.26 Mungse et al. prepared oxo-vanadium Schiff covalently anchored onto graphene oxide sheets for the oxidation of alcohols, which showed good catalytic behavior.27
In this paper, we report design and characterization of transition metal Schiff base complexes supported onto graphene oxide. Moreover, the catalytic performance of different transition metal (Fe2+, Co2+, VO2+ or Cu2+) Schiff base complexes grafted onto graphene oxide in the epoxidation of styrene using acetonitrile as solvent and tert-butyl hydroperoxide as oxidant was investigated. The obtained hybrid catalyst Cu–NH2–GO leads to a heterogeneous epoxidation of styrene, showing high conversion, high selectivity, easy recovery, and steady reuse.
Experimental
Materials and methods
Graphite power (320 mesh, 99.5%), potassium permanganate (KMnO4), concentrated sulfuric acid (H2SO4), hydrochloric acid (HCl 5%), hydrogen peroxide (H2O2 30%) 3-aminopropyltriethoxysilane (3-APTES) (Aldrich), acetylacetone, styrene (98%), Cu(CH3COO)2, Co(NO3)2·6H2O, Fe(NO3)2·9H2O, and tert-butyl hydroperoxide (TBHP 70%) were used without further treatment. Na/diphenylketone ketyl was used to dry toluene, and then the toluene was distilled under an N2 atmosphere.XRD patterns were collected on a Shimadzu XRD-6000 diffract meter equipped with Ni-filtered Cu-Ka radiation (operating at 40 kV, 30 mA). Diffractions were carried out in the 2θ ranges of 5–40° at a scanning speed of 6°/min−1. The infrared spectra were carried out on a NICOLET impact 410 spectrometer in the range of 400–4000 cm−1 after the samples were mixed with KBr and pressed into tablets. Thermo gravimetric (TG) curves were obtained on a NETZSCH STA 449C analyzer in an N2 stream in the range of 100–800 °C with a heating rate of 10 °C min−1. Scanning electron microscopy (SEM) images were obtained using a Hitachi S-4800 field emission scanning electron microscope (FE-SEM) and iridium (IXRF Systems) software. Transmission electron microscopy (TEM) photographs were taken on a Tecnai F20 (FEI Company) field emission transmission electron microscope system at an acceleration voltage of 120 kV. The specific surface area was determined using the Brunauer–Emmett–Teller (BET) equation. Raman spectra were collected from a Lab-RAM HR 800 confocal microscope Raman system using an excitation wavelength laser of 532 nm. Metal content was estimated by inductively coupled plasma atomic emission spectroscopy (ICP-AES) analysis conducted on a PerkinElmer emission spectrometer.
Synthetic procedures
The synthesis of heterogeneous catalysts was shown in Scheme 1 and described as follows.Synthesis of graphene oxide (GO)
Graphene oxide was prepared and purified by modified Hummers method.28–30 In a typical procedure, 46 ml concentrated H2SO4 was added to the graphite power (2.0 g) in a 250 ml flask, and the resulting mixture was cooled down to 0 °C in an ice bath under stirring for 2 h. Then, KMnO4 (6.0 g) was slowly added into the mixture at 0 °C. After oxidizing for 2 h, the reaction mixture was subsequently transferred to a pre-heated water bath at 35 °C and stirred for further 2 h. Then, 96 ml of deionized water was slowly added, and the temperature was increased to 95 °C. The mixture was maintained at that temperature for 15 minutes and treated by adding 30% H2O2. The resulting solid was filtered, washed by 5% HCl and deionized water and dried at 70 °C for one week. The resulting solid powder was labelled as GO.Synthesis of amino-functionalized graphene oxide (NH2–GO)
The obtained GO (1.0 g, 30 ml) was suspended in anhydrous toluene under N2 atmosphere. 3-aminopropyltrimethoxysilane (3-APTES) was grafted on graphene oxide by targeting their hydroxyl and carboxyl groups.31,32 After adding 2.3 mmol 3-APTES, the mixture was refluxed at 110 °C under nitrogen protection for 24 h. Then, the resultant solid was washed with toluene at least 3 times to remove the residual APTES. The resulting coated grapheme oxide was dried in a vacuum oven at 40 °C for 24 h, which was labeled as NH2–GO.Synthesis of copper(II) Schiff base grafted graphene oxide
0.5 g NH2–GO was dispersed in 30 ml ethanol and then 100 mg Cu(CH3COO)2 and 6 mmol acetylacetone were added. The mixture was refluxed at 70 °C for 3 h. After cooling to room temperature, the mixture was filtrated and washed with ethanol to remove the undigested Cu(CH3COO)2, followed by drying. The resulting solid powder was labeled as Cu–NH2–GO.For comparison, Fe–NH2–GO, Co–NH2–GO, and VO–NH2–GO were synthesized under the similar synthetic conditions.
Catalytic tests
The epoxidation of styrene reactions was carried out in a 10 ml round bottom flask equipped with a magnetic stirrer and reflux condenser. Typically, styrene (5 mmol), acetonitrile (5 ml) and catalyst (25 mg) were added to the reactor with appropriate amount of TBHP. Then the mixture was continuously stirred at a desired temperature in an oil bath. After reaction, the solid catalyst was filtered off, washed with acetonitrile, dried at 50 °C for 24 h. The recovered catalyst was used in order to investigate the stability and recyclability. The products of epoxidation were analyzed by a gas chromatograph (Shimadzu GC-8A) equipped with a capillary column (HJ-5, 30 M × 0.25 mm × 0.25 μm) and a FID detector.Results and discussion
IR studies
Fig. 1 shows the FT-IR spectra of the pure GO, NH2–GO, Fe–NH2–GO, Co–NH2–GO, Cu–NH2–GO and VO–NH2–GO. For pure GO, the strong band at 1731 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) represents carboxylic acid, and bands at 1053, 1224 and 1621 cm−1 are attributed to the C–O (epoxy), C–OH and C
O) represents carboxylic acid, and bands at 1053, 1224 and 1621 cm−1 are attributed to the C–O (epoxy), C–OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups in graphene oxide, respectively.33 In addition, a broad peak at 3394 cm−1 should be attributed to the stretching mode of O–H bonds, revealing the presence of many hydroxyl groups. The FTIR spectrum of NH2–GO shows some new absorption peaks at 1108 cm−1, 1037 cm−1, and 1620 cm−1 due to Si–O–Si, Si–O–C and C–N vibration, respectively.19 The appearance of these new peaks indicates the successful grafting of 3-APTES onto graphene oxide through chemical bonding. The FTIR spectra of Fe–NH2–GO, Co–NH2–GO, Cu–NH2–GO and VO–NH2–GO show a new peak at around 1610 cm−1 attributing to C
C groups in graphene oxide, respectively.33 In addition, a broad peak at 3394 cm−1 should be attributed to the stretching mode of O–H bonds, revealing the presence of many hydroxyl groups. The FTIR spectrum of NH2–GO shows some new absorption peaks at 1108 cm−1, 1037 cm−1, and 1620 cm−1 due to Si–O–Si, Si–O–C and C–N vibration, respectively.19 The appearance of these new peaks indicates the successful grafting of 3-APTES onto graphene oxide through chemical bonding. The FTIR spectra of Fe–NH2–GO, Co–NH2–GO, Cu–NH2–GO and VO–NH2–GO show a new peak at around 1610 cm−1 attributing to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching.34 In addition, the band at 1620 cm−1 due to the C–N vibration in NH2–GO almost disappears and is shifted to a lower frequency (around 1610 cm−1) in Fe–NH2–GO, Co–NH2–GO, Cu–NH2–GO and VO–NH2–GO catalysts, indicating that the chemical surroundings of nitrogen atom have been changed and almost all of the –NH2 groups have reacted with acetylacetone to form a Schiff base. Moreover, peaks in the region of 1600–1300 cm−1 are due to C–O and C–N vibrations, indicating that the Schiff base was successfully immobilized onto GO.
N stretching.34 In addition, the band at 1620 cm−1 due to the C–N vibration in NH2–GO almost disappears and is shifted to a lower frequency (around 1610 cm−1) in Fe–NH2–GO, Co–NH2–GO, Cu–NH2–GO and VO–NH2–GO catalysts, indicating that the chemical surroundings of nitrogen atom have been changed and almost all of the –NH2 groups have reacted with acetylacetone to form a Schiff base. Moreover, peaks in the region of 1600–1300 cm−1 are due to C–O and C–N vibrations, indicating that the Schiff base was successfully immobilized onto GO.
 | ||
| Fig. 1 FT-IR spectra of (a) GO, (b) NH2–GO (c) Fe–NH2–GO, (d) Co–NH2–GO (e) Cu–NH2–GO and (f) VO–NH2–GO. | ||
XRD studies
The high angle X-ray diffraction patterns of GO, NH2–GO, Fe–NH2–GO, Co–NH2–GO, Cu–NH2–GO and VO–NH2–GO are shown in Fig. 2. The pure GO shows that a sharp peak at 2θ = 11.3° corresponding to (001), which is due to the oxidation of graphite powder leading to the introduction of various oxygen-containing functional groups.27 After amino-functionalization and encapsulation of metal complex, the intensity of this peak weakens immensely and a new weak and broad peak at around 2θ = 22° is seen, which is closer to reduced graphene indicating that the major oxygen containing groups of GO have been successfully functionalized. | ||
| Fig. 2 X-ray diffraction patterns of (a) GO, (b) NH2–GO, (c) Fe–NH2–GO, (d) Co–NH2–GO (e) Cu–NH2–GO and (f) VO–NH2–GO. | ||
TEM and SEM studies
The SEM images of GO, NH2–GO and Cu–NH2–GO are shown in Fig. 3. It can be seen that GO, NH2–GO, and Cu–NH2–GO have a similar appearance with crumpling and folded feature. The SEM graphs clearly show extended sheets of lateral dimensions ranging from a few micrometers to tens of micrometers in length with layered structures.29 TEM images of GO, NH2–GO and Cu–NH2–GO are shown in Fig. 4. It reveals that the GO, NH2–GO and Cu–NH2–GO samples show typical two-dimensional crumpling structures. Additionally, the TEM images show a few stacked layers (2–3 layers) and a lateral size up to several tens of nanometers. Moreover, the TEM images show that some of the graphene layers are folded on one edge with isolated small fragments on the surfaces.TG studies
The TG curves of GO and Cu–NH2–GO are depicted in Fig. 5. The samples were heated at a constant rate of 10 °C min−1 from 100 °C to 800 °C under N2 flow. The TG curve of GO shows three distinct steps. The first weight loss up to 150 °C is due to the evaporation of absorbed water, the second weight loss observed in the range of 150–250 °C is assigned to the decomposition of labile oxygen-containing functional groups, and the third weight loss above 250 °C is attributed to the removal of more stable oxygen functionalities (such as phenol, carbonyl and quinone), which usually decompose at higher temperatures. For Cu–NH2–GO, the first region at temperatures between 100 and 250 °C corresponds to the removal of physically adsorbed water. The second region in the temperature range from 250 to 450 °C is due to undigested oxygen carrying functionalities.27 The last weight loss region above 450 °C is mainly related to the slow decomposition of Cu-Schiff base complex. Compared to the graphene oxide, the weight loss of Cu–NH2–GO is significantly lower and more residues are remained. It could be approximately deduced that the loadings of Schiff base ligand is 0.50 mmol g−1 based on the weight losses and Cu content is 0.27 mmol g−1 estimated by ICP-AES analysis. Thus, the molar ratio of ligand/metal is close to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in Cu–NH2–GO.
1 in Cu–NH2–GO.
N2 adsorption–desorption studies
The N2 adsorption–desorption isotherms of GO, NH2–GO, and Cu–NH2–GO are depicted in Fig. 6. The isotherms exhibit a typical type-IV curve and a hysteresis loop, indicating the presence of mesoporous structure. The BET surface area is 89, 26, and 23 m2g−1 for GO, NH2–GO, and Cu–NH2–GO, respectively.Raman spectroscopy studies
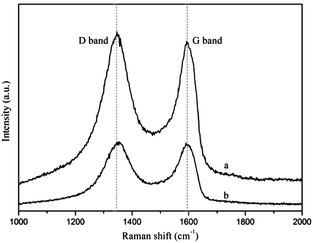
Raman spectroscopy is a widely used characterization tool to evaluate the nature of disorder on carbon-based materials. The D and G bands are the well-known characteristic of Raman spectra for carbon. The D band is related to the disorder/order degree from breathing k-point phonon of A1g symmetry while the G band is an indicator of the staking structure assigned to the E2g phonon of sp2 hybridized carbon atoms.35,36 For GO and Cu–NH2–GO, two peaks appear at around 1351 and 1591 cm−1, corresponding D band and G band, respectively. The value of ID/IG (the Raman intensity ratio of D and G bands) in Cu–NH2–GO (1.27) increases notably compared with that in the GO (1.21), indicating increased disorder in graphene lattice after functionalization of GO (Fig. 7).Catalytic properties
The heterogeneous catalyst Cu–NH2–GO was examined for epoxidation of styrene using TBHP as the oxidant in acetonitrile solvent. In order to obtain the optimum reaction condition, various parameters (reaction time, reaction temperature and the molar ratio of styrene to TBHP) influencing its catalytic property were studied.Reaction time is found to show significant effect on the styrene conversion and epoxide selectivity. The effect of reaction time on oxidation of styrene is investigated and summarized in Table 1. It is observed that the conversion of styrene gradually increases from 2 to 7 h. However, the reaction time has little effect on the epoxide selectivity (≥99.0%).
| Entry | Time (h) | Temperature (°C) | Molar ratio of Styrene/TBHP | Conversiona (%) | Selectivityb (%) | TOFc (h−1) | |
|---|---|---|---|---|---|---|---|
| So | Others | ||||||
| a Reaction conditions: catalyst 25 mg, styrene 0.67 ml, CH3CN 5 ml.b So: styrene epoxide. Others: benzaldehyde, benzoic acid, etc.c TOF, h−1: (turnover frequency) moles of substrate converted per mole metal ion per hour.d Second run.e Third run.f Forth run. | |||||||
| 1 | 2 | 80 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
50.6 | 99.0 | 1.0 | 52.2 |
| 2 | 4 | 80 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
64.2 | 99.3 | 0.7 | 66.2 |
| 3 | 6 | 80 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
85.3 | 99.1 | 0.9 | 87.9 |
| 4 | 7 | 80 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
94.3 | 99.0 | 1.0 | 97.3 |
| 5 | 7 | 80 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
44.6 | 99.4 | 0.6 | 46.0 |
| 6 | 7 | 80 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
81.9 | 99.4 | 0.6 | 84.4 |
| 7 | 7 | 40 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
18.9 | 63.9 | 36.1 | 19.5 |
| 8 | 7 | 60 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
60.1 | 90.1 | 9.9 | 61.9 |
| 9d | 7 | 80 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
95.0 | 99.4 | 0.6 | 97.9 |
| 10e | 7 | 80 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
93.6 | 99.4 | 0.6 | 96.5 |
| 11f | 7 | 80 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
93.3 | 99.3 | 0.7 | 96.2 |
The effect of styrene/oxidant molar ratio on the oxidation of styrene is shown in Table 1. Three proportions of styrene/TBHP molar ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) were considered while keeping the fixed amount of styrene (5 mmol) and catalyst (25 mg) in 5 ml of CH3CN at 80 °C. It is clear that a molar ratio of styrene to TBHP of 1
3) were considered while keeping the fixed amount of styrene (5 mmol) and catalyst (25 mg) in 5 ml of CH3CN at 80 °C. It is clear that a molar ratio of styrene to TBHP of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 is the best one to obtain the highest styrene conversion.
3 is the best one to obtain the highest styrene conversion.
Reaction temperature has a great influence on the epoxidation of styrene. The effect of reaction temperature from 40 to 80 °C on the epoxidation of styrene is summarized in Table 1. The temperature has remarkable effect on the selectivity of epoxide. High temperature is well known to be conducive to high efficiency. Amongst the three tested temperatures of 40, 60 and 80 °C, a maximum conversion of styrene and a maximum selectivity to epoxide is obtained at 80 °C.
The reusability and regeneration of Cu–NH2–GO are also investigated (shown in Table 1). The stability of the catalyst was tested by performing four reaction cycles under the same reaction conditions as described above. At the end of each reaction cycle, the catalyst was recovered by filtration, washed with acetonitrile, dried at 50 °C and then directly reused. It is evident that Cu–NH2–GO show a good recoverability without significant loss of activity or selectivity even after four catalytic cycles.
For comparison, the catalytic performances of NH2–GO, Cu(acac)2, Fe–NH2–GO, Co–NH2–GO, and VO–NH2–GO were also investigated and the reaction data are listed in Table 2. For homogeneous catalyst Cu(acac)2, it exhibits high conversion and selectivity after 7 h. Furthermore, the very low of activity of NH2–GO (9.4% of conversion) confirms that the catalytic efficiency of Cu–NH2–GO is due to the anchored coordination compound. Furthermore, other transition metal (Fe2+, Co2+, or VO2+) Schiff base complexes grafted on GO do not exhibit reasonably good activity. For example, 12.7% conversion of styrene and 88.6% selectivity to epoxide can be obtained over Co–NH2–GO. Moreover, Fe–NH2–GO shows higher catalytic activity but lower selectivity to epoxide than Co–NH2–GO. For VO–NH2–GO, its styrene conversion is slightly larger than that over Fe–NH2–GO, while its selectivity to epoxide (96%) is much higher than the latter. It should be noted that Cu–NH2–GO exhibits far better catalytic behavior than the other transition metal complexes grafted on GO.
| Catalyst | Time (h) | Conversion (%) | Selectivity (%) | Reference |
|---|---|---|---|---|
| a 10 mg catalyst.b Cu-HMS: copper(II)-containing hexagonal mesoporous silicas.c Cu-MSN: copper salicylaldimine complex grafted on mesoporous silica nanoparticles.d Cu-MCM-41: [Cu(diamine)(NO3)2] complex immobilized on MCM-41. | ||||
| Fe–NH2–GO | 7 | 30.5 | 75.8 | This study |
| Co–NH2–GO | 7 | 12.7 | 88.6 | This study |
| VO–NH2–GO | 7 | 31.1 | 96.0 | This study |
| Cu–NH2–GO | 7 | 94.3 | 99.0 | This study |
| Cu(acac)2a | 7 | 99.4 | 99.2 | This study |
| NH2–GO | 7 | 9.4 | 45.0 | This study |
| Cu-HMSb | 12 | 99 | 84 | 37 |
| Cu-MSNc | 24 | 99 | 90 | 38 |
| Cu-MCM-41d | 24 | 94 | 80 | 39 |
Table 2 lists some results over copper based heterogeneous catalysts from the literatures for comparison. It can be found that our catalyst Cu–NH2–GO is more efficient than the listed other copper based heterogeneous catalysts.37–39 Cu(II) Schiff base complex may account for the higher activity because the complex readily provides vacant coordination sites for oxygen binding. In spite of the high conversation and selectivity, Cu–NH2–GO consumes less time to complete the epoxidation reaction, which may be related to efficient synergistic effect between Cu(II) Schiff base and the GO support.
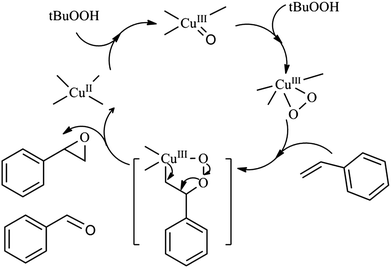
On the basis of our currently catalytic experiment results, our early work40,41 and some related literatures42,43 a propose mechanism is depicted in Scheme 2. Firstly, copper(II) complex is coordinated with TBHP to form complex comprising a higher valence transition metal-oxo compound. Secondly, copper complex transforms from CuIII-oxo to CuIII-peroxo under TBHP. Thirdly, styrene is bound with one of the metal-peroxo bonds to produce the peroxo metallocycle. Finally, the peroxo metallocycle is broken and then styrene oxide and benzaldehyde are formed and the copper(II) complex is regenerated.
Conclusions
Transition metal Schiff base complexes (Fe2+, Co2+, VO2+ or Cu2+) were successfully immobilized onto graphene oxide sheets through covalent attachment. The prepared catalysts were characterized by XRD, N2 adsorption/desorption, TEM, FT-IR, TGA, Raman and ICP-AES. The graphene oxide covalently anchored copper(II) Schiff base complex exhibited excellent catalytic performance in the epoxide of styrene. In addition, Cu–NH2–GO is very stable during the catalytic reaction and could be reused four times.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21303069), Jilin province (201105006), and Specialized Research Fund for the Doctoral Program of Higher Education (20100061120083).Notes and references
- C. Baleizao and H. Garcia, Chem. Rev., 2006, 106, 3987–4043 CrossRef CAS PubMed.
- M. Selvaraj and T. G. Lee, J. Phys. Chem. B, 2006, 110, 21793–21802 CrossRef CAS PubMed.
- S. Bhattacharjee, T. J. Dines and J. A. Anderson, J. Catal., 2004, 225, 398–407 CrossRef CAS PubMed.
- X.-T. Zhou, Q.-H. Tang and H.-B. Ji, Tetrahedron Lett., 2009, 50, 6601–6605 CrossRef CAS PubMed.
- G. Grivani, G. Bruno, H. A. Rudbari, A. D. Khalaji and P. Pourteimouri, Inorg. Chem. Commun., 2012, 18, 15–20 CrossRef CAS PubMed.
- M. G. Topuzova, S. V. Kotov and T. M. Kolev, Appl. Catal., A, 2005, 281, 157–166 CrossRef CAS PubMed.
- G. Grivani, S. Tangestaninejad, M. H. Habibi, V. Mirkhani and M. Moghadam, Appl. Catal., A, 2006, 299, 131–136 CrossRef CAS PubMed.
- M. Hatefi, M. Moghadam, I. Sheikhshoaei, V. Mirkhani, S. Tangestaninejad, I. Mohammadpoor-Baltork and H. Kargar, Appl. Catal., A, 2009, 370, 66–71 CrossRef CAS PubMed.
- Y. Yang, H. Ding, S. Hao, Y. Zhang and Q. Kan, Appl. Organomet. Chem., 2011, 25, 262–269 CrossRef CAS.
- M. Moghadam, S. Tangestaninejad, V. Mirkhani, I. Mohammadpoor-Baltork and M. Moosavifar, J. Mol. Catal. A: Chem., 2009, 302, 68–75 CrossRef CAS PubMed.
- I. Kuźniarska-Biernacka, C. Pereira, A. P. Carvalho, J. Pires and C. Freire, Appl. Clay Sci., 2011, 53, 195–203 CrossRef PubMed.
- P. Visuvamithiran, M. Palanichamy, K. Shanthi and V. Murugesan, Appl. Catal., A, 2013, 462–463, 31–38 CrossRef CAS PubMed.
- R. I. Kureshy, N.-u. H. Khan, S. H. R. Abdi, I. Ahmad, S. Singh and R. V. Jasra, J. Catal., 2004, 221, 234–240 CrossRef CAS PubMed.
- S. Dorbes, C. Pereira, M. Andrade, D. Barros, A. M. Pereira, S. L. H. Rebelo, J. P. Araújo, J. Pires, A. P. Carvalho and C. Freire, Microporous Mesoporous Mater., 2012, 160, 67–74 CrossRef CAS PubMed.
- F. Esnaashari, M. Moghadam, V. Mirkhani, S. Tangestaninejad, I. Mohammadpoor-Baltork, A. R. Khosoropour, M. Zakeri and S. Hushmandrad, Polyhedron, 2012, 48, 212–220 CrossRef CAS PubMed.
- A. Kaniyoor, R. Imran Jafri, T. Arockiadoss and S. Ramaprabhu, Nanoscale, 2009, 1, 382 RSC.
- X. Yang, X. Zhang, Y. Ma, Y. Huang, Y. Wang and Y. Chen, J. Mater. Chem., 2009, 19, 2710 RSC.
- J. Liu, H. Bai, Y. Wang, Z. Liu, X. Zhang and D. D. Sun, Adv. Funct. Mater., 2010, 20, 4175–4181 CrossRef CAS.
- R. Nie, J. Wang, L. Wang, Y. Qin, P. Chen and Z. Hou, Carbon, 2012, 50, 586–596 CrossRef CAS PubMed.
- J. M. Yun, J. S. Yeo, J. Kim, H. G. Jeong, D. Y. Kim, Y. J. Noh, S. S. Kim, B. C. Ku and S. I. Na, Adv. Mater., 2011, 23, 4923–4928 CrossRef CAS PubMed.
- Y. Zhu, S. Murali, W. Cai, X. Li, J. W. Suk, J. R. Potts and R. S. Ruoff, Adv. Mater., 2010, 22, 3906–3924 CrossRef CAS PubMed.
- S. Park, K.-S. Lee, G. Bozoklu, W. Cai, S. T. Nguyen and R. S. Ruoff, ACS Nano, 2008, 2, 572–578 CrossRef CAS PubMed.
- D. R. Dreyer, S. Park, C. W. Bielawski and R. S. Ruoff, Chem. Soc. Rev., 2010, 39, 228 RSC.
- T. Szabo, O. Berkesi, P. Forgo, K. Josepovits, Y. Sanakis, D. Petridis and I. Dekany, Chem. Rev., 2006, 18, 2740–2749 CAS.
- O. C. Compton and S. T. Nguyen, Small, 2010, 6, 711–723 CrossRef CAS PubMed.
- G. M. Scheuermann, L. Rumi, P. Steurer, W. Bannwarth and R. Mülhaupt, J. Am. Chem. Soc., 2009, 131, 8262–8270 CrossRef CAS PubMed.
- H. P. Mungse, S. Verma, N. Kumar, B. Sain and O. P. Khatri, J. Mater. Chem., 2012, 22, 5427 RSC.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- Y. Lin, J. Jin and M. Song, J. Mater. Chem., 2011, 21, 3455 RSC.
- G. I. Titelman, V. Gelman, S. Bron, R. L. Khalfin, Y. Cohen and H. Bianco-Peled, Carbon, 2005, 43, 641–649 CrossRef CAS PubMed.
- H. Wang, J. Huang, S. Wu, C. Xu, L. Xing, L. Xu and Q. Kan, Mater. Lett., 2006, 60, 2662–2665 CrossRef CAS PubMed.
- Q. Zhao, D. Chen, Y. Li, G. Zhang, F. Zhang and X. Fan, Nanoscale, 2013, 5, 882–885 RSC.
- D. Pan, S. Wang, B. Zhao, M. Wu, H. Zhang, Y. Wang and Z. Jiao, Chem. Mater., 2009, 21, 3136–3142 CrossRef CAS.
- S. Dorbes, C. Pereira, M. Andrade, D. Barros, A. M. Pereira, S. L. H. Rebelo, J. P. Araújo, J. Pires, A. P. Carvalho and C. Freire, Microporous Mesoporous Mater., 2012, 160, 67–74 CrossRef CAS PubMed.
- Z. G. Le, Z. Liu, Y. Qian and C. Wang, Appl. Surf. Sci., 2012, 258, 5348–5353 CrossRef CAS PubMed.
- F. Tuinstra and J. L. Koenig, J. Chem. Phys., 1970, 53, 1126–1130 CrossRef CAS PubMed.
- X. Lu and Y. Yuan, Appl. Catal., A, 2009, 365, 180–186 CrossRef CAS PubMed.
- D. Tang, W. Zhang, Y. Zhang, Z.-A. Qiao, Y. Liu and Q. Huo, J. Colloid Interface Sci., 2011, 356, 262–266 CrossRef CAS PubMed.
- S. Jana, S. Bhunia, B. Dutta and S. Koner, Appl. Catal., A, 2011, 392, 225–232 CrossRef CAS PubMed.
- Z. Li, S. Wu, Y. Ma, H. Liu, J. Hu, L. Liu, Q. Huo, J. Guan and Q. Kan, Transition Met. Chem., 2013, 38, 243–251 CrossRef CAS.
- Z. Li, H. Ding, S. Wu, H. Liu, H. Su, J. Sun, D. Zheng, Q. Huo, J. Guan and Q. Kan, Mater. Res. Bull., 2013, 48, 1920–1926 CrossRef CAS PubMed.
- A. Modaka, M. Nandi and A. Bhaumik, Catal. Today, 2012, 198, 45–51 CrossRef PubMed.
- G. Grivani, A. D. Khalaji, V. Tahmasebi, K. Gotoh and H. Ishida, Polyhedron, 2012, 31, 265–271 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |