Conformationally restricted glutamic acid analogues: stereoisomers of 1-aminospiro[3.3]heptane-1,6-dicarboxylic acid†
Anton V. Chernykhab,
Dmytro S. Radchenkoac,
Oleksandr O. Grygorenkoc,
Dmitriy M. Volochnyukb,
Svitlana V. Shishkinad,
Oleg V. Shishkind and
Igor V. Komarov*c
aEnamine Ltd., Alexandra Matrosova Street 23, Kyiv 01103, Ukraine
bInstitute of Organic Chemistry, National Academy of Sciences of Ukraine, Murmanska 5, Kyiv 02660, Ukraine
cTaras Shevchenko National University of Kyiv, Volodymyrska Street 60, Kyiv 01601, Ukraine. E-mail: ik214@yahoo.com
dSTC “Institute for Single Crystals”, National Academy of Sciences of Ukraine, 60 Lenina ave., 61001 Kharkiv, Ukraine
First published on 3rd February 2014
Abstract
All four stereoisomers of the title compound (1a–d) were prepared, starting from a common precursor, 3-oxocyclobutanecarboxylic acid. Lewis acid-catalyzed rearrangement of a 8-oxadispiro[2.0.3.1]octane-6-carboxylic acid derivative was used as the key synthetic step to construct the suitably functionalized spiro[3.3]heptane skeleton. A stabilized oxaphosphetane intermediate of the Wittig reaction was detected along the synthetic route. Separation of the diastereomeric intermediates allowed each target compound to be obtained as a single stereoisomer. The target compounds are all analogues of the glutamic acid; they mimic glutamate in a large array of restricted conformations, which might be used in mechanistic studies or in a systematic search for biologically active compounds.
Introduction
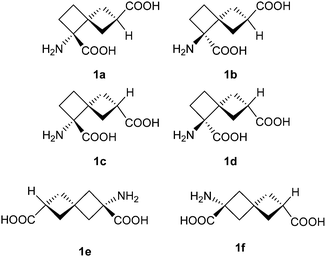
Sets of isomeric functionalized derivatives of small conformationally restricted molecular frameworks (scaffolds) were shown to be useful in design of the molecules used to map receptor binding sites1,2 and in a systematic search for leads in drug design.3–5 A term “stereolibrary” was coined for such compound sets where only the relative position of the functional groups in space varies while the molecular topology is the same among all members of the sets.6 Spirocyclic scaffolds are especially attractive for the design of the stereolibraries, as they might ensure vide variety of spatial disposition of the functional groups, and consequently, allow constructing chemically diverse compound sets.7–10 For example, based on the spiro[3.3]heptane scaffold, one can construct nine enantiomeric pairs of rigid glutamic acid analogues, which differ in position and relative orientation of the carboxylic and aminocarboxylate moieties.11 Conformationally restricted glutamic acid analogues of such type might be used to map the glutamate receptor binding sites, in mechanistic studies of the enzymes which act on glutamate, and ultimately, in the search for biologically active compounds using different systematic approaches.6,12–15 In this paper we report on the synthesis and stereochemical assignment of novel spiro[3.3]heptane-based glutamic acid analogues – the stereolibrary composed of 1-aminospiro[3.3]heptane-1,6-dicarboxylic acids 1a–d.Results and discussion
By now, synthesis of only two of the eighteen theoretically possible members of the spiro[3.3]heptane-based library of the glutamate analogues was reported,11 namely, (aS)- and (aR)-2-amino-spiro[3.3]heptane-2,6-dicarboxylic acids (1e and 1f, respectively). Their synthesis was based on simple malonate chemistry, but cannot be adapted to the 1,6-isomers. Completely different strategy was chosen in the present work, highlighted by the retrosynthetic analysis shown in the Scheme 1.In our approach to compounds 1a–d, a modified Strecker reaction was proposed for construction of the aminocarboxylate moiety in the late steps of the synthesis. Corresponding retrosynthetic transformation led to the ketoester 2 – a key intermediate of the synthesis. Compound 2, in turn, could be obtained by rearrangement of the epoxide 3. Oxaspiro[2.2]pentanes (like 3) can be prepared either by Corey–Chaykovsky reaction with cyclopropyldiphenyl sulfonium ylide16 or by epoxidation of the corresponding alkene (4), which can be obtained by Wittig olefination with phosphorus ylide 6.17 Both approaches led to the ketoester 5 as the starting material, which is readily accessible from commercially available 3-oxocyclobutanecarboxylic acid (7). Benzyl group (R in the Scheme 1) would be an optimal choice for the protection of the carboxylic moiety: it would diminish the volatility of the intermediate alkene 4, as well as allow for UV detection of the intermediate products upon chromatographic purifications.
The retrosynthetic scheme should consider a possibility to isolate all four target compounds 1a–d as the single stereoisomers. To achieve this, we decided to use separation of diastereomeric intermediates at the appropriate stages of the synthesis. Compound 2 could be obtained as a mixture of diastereomers, so the first separation could be done at this step. Resolution of enantiomers of the amino acid precursors synthesized further on could be achieved by the use of a chiral auxiliary; Strecker reaction with a chiral amine (e.g. (S)-phenylglycinol) proved to be an efficient tool for that purpose in the cyclobutane series.18
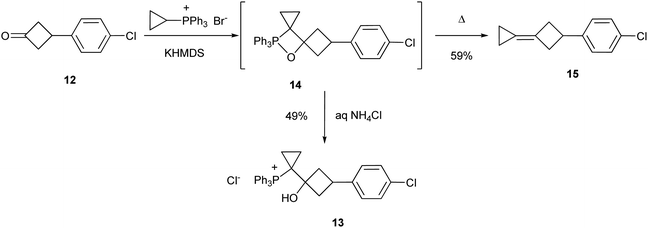
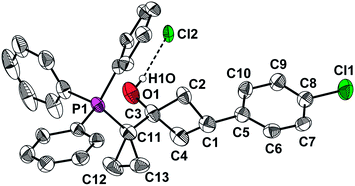
Implementation of the above retrosynthetic plan started with esterification of the acid 7 via the corresponding acid chloride (Scheme 2). The method for the synthesis of cyclobutanones reported by Carreira et al.,19 based on the Corey–Chaykovsky reaction of 8 with cyclopropyldiphenyl sulfonium ylide (KHMDS, THF, −40 °C, 4 h, then LiI, 50 °C, 15 h, one-pot) failed in our hands, a very complex mixture of unidentified compounds was isolated. Therefore, we explored an alternative sequence based on the Wittig olefination with ylide 6. Our initial attempts to introduce the ketoester 8 into this transformation were also unfruitful. Therefore, we performed a more detailed study of this reaction using ketone 12 as the model substrate. This ketone and its olefination product turned to be not volatile and did not contain ester moiety which complicated optimization of the reaction conditions with the ketoester 8. Reaction of 12 under the standard Wittig olefination conditions gave an unexpected product 13 (Scheme 3), the structure of which was determined by an X-ray diffraction study (Fig. 1). Presumably, the salt 13 formed by protonation of the corresponding oxaphosphetane 14 upon the work-up of the reaction mixture with aqueous NH4Cl. Oxaphosphetanes are usually unstable; the increased stability of 14 towards decomposition to alkene 15 and triphenylphosphine oxide can be explained by high steric strain in the molecule of 15. It should be noted that although examples of stable oxaphosphetanes are known in the literature,20 in all the cases reported to date the stabilization was achieved through electronic effects.
Transformation of 14 into the alkene 15 was achieved at elevated temperature by heating at reflux in THF. Application of these conditions to the ketoester 8 led to the formation of alkene 9, which was isolated in 46% yield. Epoxidation of 9 with MCBPA was accompanied by partial rearrangement of the epoxide 10 to ketone 11. Therefore, compound 10 was not isolated but treated with BF3·Et2O to give a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
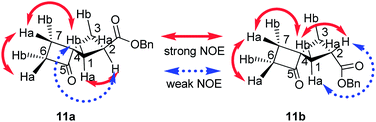
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of diastereomers 11a and 11b, which were separated by column chromatography. The stereochemical configuration of 11a,b was established by 1D- and 2D-NOESY experiments (Fig. 2, see the ESI† for details).
1 mixture of diastereomers 11a and 11b, which were separated by column chromatography. The stereochemical configuration of 11a,b was established by 1D- and 2D-NOESY experiments (Fig. 2, see the ESI† for details).
Reaction of 11a with (S)-phenylglycinol under various conditions followed by treatment with TMSCN–MeOH was accompanied by partial transesterification and resulted in an equilibrium mixture of the corresponding aminonitriles and cyanohydrins with COOBn (68%) and COOMe (32%) ester groups, which were difficult to separate. Therefore, we used Ti(Oi-Pr)4 in i-PrOH as water scavenger and mild Lewis acidic catalyst for the first step of the reaction, i.e. imine formation. The reaction was also accompanied by transesterification; after addition of TMSCN, a mixture of diastereomers 16a and 16b was obtained (Scheme 4). The diastereomers were separated by column chromatography. Compounds 16a and 16b appeared to be unstable: formation of the starting ketone 11a and epimerization were observed upon standing for a few days. Therefore, both 16a and 16b were subjected to the next step immediately after the separation. Compounds 16c and 16d were obtained from 11b in an analogous manner.
Further transformation of 16a–d included cleavage of the (S)-phenylglycinol residue with Pb(OAc)4 (Scheme 5). The intermediate imines 17a–d were not isolated, and immediately subjected to hydrolysis. Under reflux in aqueous HCl, hydrolysis of the nitrile moiety in 16 was too slow presumably due to considerable steric hindrance. Therefore, we used a three-step reaction sequence including formation of imidoyl chloride (HCl–CH2Cl2), imidoester (HCl–MeOH), and final hydrolysis to carboxylic acid (aqueous HCl). Imine and ester moieties were also hydrolyzed at this step to give amino acids 1a–d, which were isolated as hydrochlorides in 70–83% yields (based on 16a–d).
In order to determine the absolute configuration of the aminonitriles 16a–d, tricyclic derivatives 18a–d were synthesized (Scheme 6) via imidoester formation followed by cyclization. After the work-up of the reaction mixture, a mixture of 18 and methyl esters 19 were obtained; in the case of 16c, the corresponding ester 19c was isolated and characterized. No substantial transformation of 19c to 18c was observed during prolonged heating in toluene. Therefore, the tricyclic derivatives 18a–d were separated from the corresponding esters 19 by column chromatography. Crystals suitable for X-ray diffraction studies was obtained for compounds 18a–c. The results of their X-ray crystallographic analysis, combined with the 1D- and 2D-NOESY experiments for the ketoesters 11a and 11b mentioned above allowed us to deduce eventually the stereoconfiguration of final spirocyclic glutamic acid analogues 1a–d (see the ESI† for details in structural and stereochemical assignments of compounds 11a,b by 1D and 2D-NOESY experiments, compounds 18a–c by X-ray crystallography, and discussion).
Conclusions
The Lewis acid-catalyzed rearrangement of a 8-oxadispiro [2.0.3.1]octane skeleton proved to be efficient for constructing 1,6-functionalised spiro[3.3]heptanes. Using this strategy, rigid spirocyclic glutamic acid analogues 1a–d were synthesized in eight laboratory steps, starting from the common precursor, 3-oxocyclobutanecarboxylic acid. Separation of the diastereomeric intermediates along the synthetic pathway allowed isolation of the target compounds as the single enantiomers. The synthesized stereolibrary 1a–d might be useful as a tool in mechanistic studies of enzymes or receptors for which glutamic acid is the substrate or the ligand, respectively. Easy functionalization of the spiro[3.3]heptane scaffold might also be of use in medicinal chemistry, in the systematic search for biologically active compounds derived from this three-dimensional molecular framework.Experimental section
General
Solvents were purified according to the standard procedures. Compound 7 was purchased from commercial sources; compound 12 (ref. 21) and cyclopropyltriphenylphosphonium bromide22 were prepared using the procedures reported in the literature. Melting points were measured on an automated melting point system. Analytical TLC was performed using Polychrom SI F254 plates. Column chromatography was performed using silica gel (230–400 mesh) as the stationary phase. 1H, 13C NMR, and all 2D NMR spectra were recorded at 499.9 or 400.4 MHz for protons and 124.9 or 100.4 MHz for carbon-13. Chemical shifts are reported in ppm downfield from TMS (1H, 13C) as an internal standard. MS analyses were done on an LCMS instrument with chemical ionization (CI) or GCMS instrument with electron impact ionization (EI).Benzyl 3-oxocyclobutane-1-carboxylate (8)
To a mixture of benzyl alcohol (113 mL, 1.09 mol) and triethylamine (122 mL, 0.874 mol) in CH2Cl2 (400 mL), a solution of 3-oxocyclobutanecarbonyl chloride23 (57.9 g, 0.437 mol) in CH2Cl2 (200 mL) was added at 5 °C. The resulting solution was stirred at room temperature for 15 min, and then poured into water. Organic phase was washed with 10% aq. citric acid, saturated aq. NaHCO3, brine, dried over Na2SO4, filtered, evaporated, and distilled in vacuo. The unreacted benzyl alcohol was distilled off first (50 °C/1 mmHg), followed by benzyl 3-oxocyclobutanecarboxylate 8 (100 °C/1 mmHg). The yield was 35.2 g (0.172 mol, 40%). Yellow oil. Bp 100 °C (1 mmHg). 1H NMR (500 MHz, CDCl3) 3.19–3.34 (m, 3H), 3.34–3.49 (m, 2H), 5.20 (s, 2H), δ 7.29–7.45 (m, 5H). 13C NMR (126 MHz, CDCl3) δ 27.4, 51.6, 67.0, 128.3, 128.5, 128.6, 135.5, 173.8, 203.4. MS (GCMS) 204 (M+), 91 (C7H7+). Anal. calcd for C12H12O3 C 70.58, H 5.92. Found C 70.36, H 6.17.(1-(3-(4-Chlorophenyl)-1-hydroxycyclobutyl)cyclopropyl)triphenylphosphonium chloride (13)
To a suspension of cyclopropyltriphenylphosphonium bromide (2.19 g, 5.71 mmol) in THF (25 mL), KHMDS (0.5 M in toluene, 12.6 mL, 6.28 mmol) was added at −30 °C under an argon atmosphere. The orange solution was stirred for 2 h at rt, and then a solution of 3-(4-chlorophenyl)cyclobutanone 12 (0.928 g, 5.14 mmol) in THF (10 mL) was slowly added at −78 °C. The reaction mixture was stirred at −78 °C for 1 h, warmed up to rt, stirred overnight, and then poured into cold saturated aq. NH4Cl. The mixture was extracted with CHCl3, the organic phase was dried over Na2SO4, filtered and evaporated. The crude product was recrystallized from EtOAc–CH3CN, the precipitate of (1-(3-(4-chlorophenyl)-1-hydroxycyclobutyl)cyclopropyl)triphenylphosphonium chloride 13 was filtered and washed with EtOAc. The yield was 1.46 g (2.81 mmol, 49%). White solid. Mp 139–140 °C. 1H NMR (500 MHz, CDCl3) δ 1.04 (d, J = 15.7 Hz, 2H), 1.81 (d, J = 7.2 Hz, 2H), 2.37 (t, J = 10.7 Hz, 2H), 2.48–2.63 (m, 2H), 2.78–2.90 (m, 1H), 7.12–7.18 (m, 2H), 7.24–7.33 (m, 2H), 7.45 (br s, 1H), 7.60–7.68 (m, 6H), 7.69–7.78 (m, 3H), 7.87–8.01 (m, 6H). 13C NMR (126 MHz, CDCl3) δ 10.0, 22.4 (d, J = 77.2 Hz), 32.2, 43.4 (d, J = 4.7 Hz), 73.3 (d, J = 2.2 Hz), 119.7 (d, J = 87.1 Hz), 128.4, 128.8, 129.8 (d, J = 12.5 Hz), 131.8, 134.5 (d, J = 2.8 Hz), 135.5 (d, J = 9.5 Hz), 143.2. MS (LCMS) 484/486 (MH+ − Cl−). Anal. calcd for C31H29Cl2OP·CH3CN C 70.72, H 5.75, Cl 12.65, N 2.50. Found C 70.39, H 5.71, Cl 12.43, N 2.74.Chloro-4-(3-cyclopropylidenecyclobutyl)benzene (15)
To a suspension of cyclopropyltriphenylphosphonium bromide (2.67 g, 6.96 mmol) in THF (30 mL), KHMDS (0.5 M in toluene, 15.3 mL, 7.65 mmol) was added at −30 °C under argon atmosphere. The orange solution was stirred at rt for 2 h, and then a solution of 3-(4-chlorophenyl)cyclobutanone 12 (1.13 g, 6.26 mmol) in THF (57 mL) was added slowly at −78 °C. The reaction mixture was stirred at −78 °C for 1 h, then warmed up to rt, stirred overnight, refluxed for 3 h, and poured into cold saturated aq. NH4Cl. The mixture was extracted with EtOAc, the organic phase was dried over Na2SO4, filtered and evaporated. The brown oil was treated with hexane, the solution was decanted and evaporated. The crude product was purified by column chromatography (hexane as an eluent). The yield was 0.75 g (3.66 mmol, 59%). Colorless oil. TLC: Rf = 0.58 (hexanes; UV). 1H NMR (500 MHz, CDCl3) δ 1.07 (d, J = 1.9 Hz, 4H), 2.85–2.98 (m, 2H), 3.15–3.29 (m, 2H), 3.61 (quint, J = 8.2 Hz, 1H), 7.25 (d, J = 8.1 Hz, 2H), 7.30 (d, J = 8.1 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 2.3, 35.3, 39.2, 77.2, 111.7, 123.7, 128.0, 128.5, 131.7, 144.7. MS (GCMS) 204/206 (M+), 169 (M+ − Cl−). Anal. calcd for C13H13Cl C 76.28, H 6.40, Cl 17.32. Found C 76.66, H 6.21, Cl 17.39.Benzyl 3-cyclopropylidenecyclobutanecarboxylate (9)
To a suspension of cyclopropyltriphenylphosphonium bromide (50.6 g, 0.132 mol) in THF (570 mL), KHMDS (0.5 M in toluene, 290 mL, 0.145 mol) was added at −30 °C under argon atmosphere. The orange solution was stirred for 2 h at rt and then a solution of benzyl 3-oxocyclobutane-1-carboxylate 8 (24.2 g, 0.119 mol) in THF (200 mL) was added slowly at −78 °C. The reaction mixture was stirred at −78 °C for 1 h, warmed up to rt, stirred overnight, refluxed for 3h, and then poured into cold saturated aq. NH4Cl. The mixture was extracted with EtOAc, the organic phase was dried over Na2SO4, filtered and evaporated. The brown oil was treated and decanted with hexane–EtOAc (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (3 × 110 mL), and the combined organic extracts were evaporated. The crude product was purified form by column chromatography (hexanes–EtOAc (20
1) (3 × 110 mL), and the combined organic extracts were evaporated. The crude product was purified form by column chromatography (hexanes–EtOAc (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as an eluent). The yield was 12.5 g (54.8 mmol, 46%). Colorless oil. TLC: Rf = 0.435 (hexanes–EtOAc 20
1) as an eluent). The yield was 12.5 g (54.8 mmol, 46%). Colorless oil. TLC: Rf = 0.435 (hexanes–EtOAc 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; UV). 1H NMR (500 MHz, CDCl3) δ 1.03 (s, 4H), 2.97–3.07 (m, 2H), 3.07–3.18 (m, 2H), 3.21–3.33 (m, 1H), 5.18 (s, 2H), 7.28–7.44 (m, 5H). 13C NMR (126 MHz, CDCl3) δ 2.2, 34.0, 34.8, 66.3, 112.5, 122.6, 128.2, 128.2, 128.6, 136.2, 175.2. MS (GCMS) 228 (M+), 137 (M+ − C7H7), 91 (C7H7+). Anal. calcd for C15H16O2 C 78.92, H 7.06. Found C 79.10, H 7.36.
1; UV). 1H NMR (500 MHz, CDCl3) δ 1.03 (s, 4H), 2.97–3.07 (m, 2H), 3.07–3.18 (m, 2H), 3.21–3.33 (m, 1H), 5.18 (s, 2H), 7.28–7.44 (m, 5H). 13C NMR (126 MHz, CDCl3) δ 2.2, 34.0, 34.8, 66.3, 112.5, 122.6, 128.2, 128.2, 128.6, 136.2, 175.2. MS (GCMS) 228 (M+), 137 (M+ − C7H7), 91 (C7H7+). Anal. calcd for C15H16O2 C 78.92, H 7.06. Found C 79.10, H 7.36.
Benzyl (2s,4r)-5-oxospiro[3.3]heptane-2-carboxylate (11a) and benzyl (2r,4s)-5-oxospiro[3.3]heptane-2-carboxylate (11b)
To a cooled (0 °C) solution of benzyl 3-cyclopropylidenecyclo butanecarboxylate 9 (8.5 g, 37.2 mmol) in CH2Cl2 (160 mL), a solution of meta-chloroperoxybenzoic acid (9.83 g, 85% purity, 48.4 mmol) in CH2Cl2 (150 mL) was added dropwise. The reaction was monitored by TLC (EtOAc–Hex 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). After the reaction was complete (ca. 30 min), the mixture was washed with 10% aq. Na2SO3 (twice) and saturated aq. NaHCO3, dried over Na2SO4, filtered and evaporated. The resulting oil was dissolved in dry diethyl ether and cooled to 0 °C. BF3·Et2O (0.53 g, 3.73 mmol) was added slowly to the solution dropwise, and the mixture was stirred at 0 °C for 15 min. The organic layer was washed with saturated aq. NaHCO3 and brine, dried over MgSO4 and evaporated to give a crude mixture of two diastereomers 11a,b at 3
10). After the reaction was complete (ca. 30 min), the mixture was washed with 10% aq. Na2SO3 (twice) and saturated aq. NaHCO3, dried over Na2SO4, filtered and evaporated. The resulting oil was dissolved in dry diethyl ether and cooled to 0 °C. BF3·Et2O (0.53 g, 3.73 mmol) was added slowly to the solution dropwise, and the mixture was stirred at 0 °C for 15 min. The organic layer was washed with saturated aq. NaHCO3 and brine, dried over MgSO4 and evaporated to give a crude mixture of two diastereomers 11a,b at 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio (NMR data). Both isomers were obtained in pure form by column chromatography (cyclohexane–EtOAc (4
2 ratio (NMR data). Both isomers were obtained in pure form by column chromatography (cyclohexane–EtOAc (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as an eluent). The chromatographic separation yielded 4.47 g (18.3 mmol, 49%) of trans-isomer 11a (eluted first) and 2.27 g (9.29 mmol, 25%) of cis-isomer 11b.
1) as an eluent). The chromatographic separation yielded 4.47 g (18.3 mmol, 49%) of trans-isomer 11a (eluted first) and 2.27 g (9.29 mmol, 25%) of cis-isomer 11b.
11a: colorless oil. TLC: Rf = 0.52 (cyclohexane–EtOAc 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; UV). 1H NMR (400 MHz, CDCl3) δ 1.97 (t, J = 8.5 Hz, H7a, H7b), 2.33 (t, J = 10.6 Hz, H1b, H3b), 2.54 (t, J = 10.6 Hz, H1a, H3a), 2.88 (t, J = 8.5 Hz, H6a, H6b), 3.15 (quint, J = 8.6 Hz, H2), 5.06 (s, CH2Ph), 7.19–7.38 (m, C6H5). 13C NMR (126 MHz, CDCl3) δ 24.4 (C7), 32.8 (C2), 32.9 (C1, C3), 43.1 (C6), 60.1 (C4), 66.4 (CH2Ph), 128.1 (C6H5), 128.3 (C6H5), 128.6 (C6H5), 136.0 (C6H5), 174.3 (COOBn), 213.4 (C5). MS (GCMS) 244 (M+), 91 (C7H7+). Anal. calcd. for C15H16O3 C 73.75, H 6.60. Found C 73.94, H 6.66.
1; UV). 1H NMR (400 MHz, CDCl3) δ 1.97 (t, J = 8.5 Hz, H7a, H7b), 2.33 (t, J = 10.6 Hz, H1b, H3b), 2.54 (t, J = 10.6 Hz, H1a, H3a), 2.88 (t, J = 8.5 Hz, H6a, H6b), 3.15 (quint, J = 8.6 Hz, H2), 5.06 (s, CH2Ph), 7.19–7.38 (m, C6H5). 13C NMR (126 MHz, CDCl3) δ 24.4 (C7), 32.8 (C2), 32.9 (C1, C3), 43.1 (C6), 60.1 (C4), 66.4 (CH2Ph), 128.1 (C6H5), 128.3 (C6H5), 128.6 (C6H5), 136.0 (C6H5), 174.3 (COOBn), 213.4 (C5). MS (GCMS) 244 (M+), 91 (C7H7+). Anal. calcd. for C15H16O3 C 73.75, H 6.60. Found C 73.94, H 6.66.
11b: colorless oil. TLC: Rf = 0.45 (cyclohexane–EtOAc 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; UV). 1H NMR (400 MHz, CDCl3) δ 2.11 (t, J = 8.4 Hz, H7a, H7b), 2.19–2.34 (m, H1b, H3b), 2.63–2.77 (m, H1a, H3a), 2.89 (t, J = 8.5 Hz, H6a, H6b), 3.00–3.15 (m, H2), 5.08 (s, CH2Ph), 7.21–7.36 (m, C6H5). 13C NMR (126 MHz, CDCl3) δ 25.7 (C7), 31.9 (C2), 33.3 (C1, C3), 43.2 (C6), 59.5 (C4), 66.3 (CH2Ph), 128.1 (C6H5), 128.2 (C6H5), 128.5 (C6H5), 135.9 (C6H5), 173.7 (COOBn), 210.8 (C5). MS (GCMS) 244 (M+), 91 (C7H7+). Anal. calcd for C15H16O3 C 73.75, H 6.60. Found C 73.51, H 6.38.
1; UV). 1H NMR (400 MHz, CDCl3) δ 2.11 (t, J = 8.4 Hz, H7a, H7b), 2.19–2.34 (m, H1b, H3b), 2.63–2.77 (m, H1a, H3a), 2.89 (t, J = 8.5 Hz, H6a, H6b), 3.00–3.15 (m, H2), 5.08 (s, CH2Ph), 7.21–7.36 (m, C6H5). 13C NMR (126 MHz, CDCl3) δ 25.7 (C7), 31.9 (C2), 33.3 (C1, C3), 43.2 (C6), 59.5 (C4), 66.3 (CH2Ph), 128.1 (C6H5), 128.2 (C6H5), 128.5 (C6H5), 135.9 (C6H5), 173.7 (COOBn), 210.8 (C5). MS (GCMS) 244 (M+), 91 (C7H7+). Anal. calcd for C15H16O3 C 73.75, H 6.60. Found C 73.51, H 6.38.
Isopropyl (2S,4r,5R)-5-cyano-5-(((S)-2-hydroxy-1-phenyl ethyl)amino)spiro[3.3]heptane-2-carboxylate (16a) and isopropyl (2R,4r,5S)-5-cyano-5-(((S)-2-hydroxy-1-phenyl ethyl)amino)spiro[3.3]heptane-2-carboxylate (16b)
Compound 11a (1.50 g, 6.14 mmol), S-α-phenylglycinol (1.01 g, 7.37 mmol) and 2-propanol (20 mL) were placed into a 50 mL, two-necked flask equipped with a magnetic stirrer and calcium chloride drying tube. Titanium isopropoxide (4.36 g (4.57 mL), 15.35 mmol) was added to the solution. After stirring at rt for 3 h, TMSCN (1.83 g, 2.46 mL, 18.45 mmol; caution – toxic! perform all the operations under a fumehood!) was added. The reaction mixture was stirred at rt overnight, and then poured into EtOAc (350 mL). The mixture was diluted with water (350 mL), shaken, and the precipitate was filtered. The organic layer was dried over Na2SO4 and evaporated to give a crude mixture of two diastereomers (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 by NMR). Separation of isomers by column chromatography (cyclohexane–EtOAc (2
2 by NMR). Separation of isomers by column chromatography (cyclohexane–EtOAc (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as an eluent) yielded a mixture of 16a with benzyl alcohol (eluted first) (1.25 g, 84% purity by NMR, 3.07 mmol, 50% yield) and a pure 16b (eluted second) (0.50 g, 1.46 mmol, 24%). Compound 16a was used in the next step without any additional purification.
1) as an eluent) yielded a mixture of 16a with benzyl alcohol (eluted first) (1.25 g, 84% purity by NMR, 3.07 mmol, 50% yield) and a pure 16b (eluted second) (0.50 g, 1.46 mmol, 24%). Compound 16a was used in the next step without any additional purification.
16a: yellow oil. TLC: Rf = 0.46 (cyclohexane–EtOAc 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; UV). 1H NMR (500 MHz, CDCl3) δ 1.24 (d, J = 6.2 Hz, 6H), 1.38–1.48 (m, 1H), 1.61–1.72 (m, 1H), 1.84–1.96 (m, 2H), 2.14 (dd, J = 12.0, 6.7 Hz, 1H), 2.29 (dd, J = 12.3, 6.8 Hz, 1H), 2.41 (br s, 2H), 2.55–2.66 (m, 1H), 2.85 (t, J = 9.7 Hz, 1H), 3.03–2.92 (m, 1H), 3.52–3.64 (m, 1H), 3.76 (dd, J = 11.0, 4.0 Hz, 1H), 4.05 (dd, J = 9.1, 4.0 Hz, 1H), 4.95–5.06 (m, 1H), 7.22–7.44 (m, 5H). 13C NMR (126 MHz, CDCl3) δ 21.8, 29.4, 30.3, 30.7, 33.2, 34.2, 46.9, 60.8, 62.4, 67.1, 68.0, 121.2, 127.8, 128.1, 128.6, 140.6, 174.7. MS (LCMS) 343 (MH+).
1; UV). 1H NMR (500 MHz, CDCl3) δ 1.24 (d, J = 6.2 Hz, 6H), 1.38–1.48 (m, 1H), 1.61–1.72 (m, 1H), 1.84–1.96 (m, 2H), 2.14 (dd, J = 12.0, 6.7 Hz, 1H), 2.29 (dd, J = 12.3, 6.8 Hz, 1H), 2.41 (br s, 2H), 2.55–2.66 (m, 1H), 2.85 (t, J = 9.7 Hz, 1H), 3.03–2.92 (m, 1H), 3.52–3.64 (m, 1H), 3.76 (dd, J = 11.0, 4.0 Hz, 1H), 4.05 (dd, J = 9.1, 4.0 Hz, 1H), 4.95–5.06 (m, 1H), 7.22–7.44 (m, 5H). 13C NMR (126 MHz, CDCl3) δ 21.8, 29.4, 30.3, 30.7, 33.2, 34.2, 46.9, 60.8, 62.4, 67.1, 68.0, 121.2, 127.8, 128.1, 128.6, 140.6, 174.7. MS (LCMS) 343 (MH+).
16b: yellow oil. TLC: Rf = 0.34 (cyclohexane–EtOAc 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; UV). 1H NMR (500 MHz, CDCl3) δ 1.21 (d, J = 6.2 Hz, 6H), 1.96–2.25 (m, 8H), 2.32–2.41 (m, 1H), 2.42–2.54 (m, 1H), 2.67–2.54 (m, 1H), 3.60–3.70 (m, 1H), 3.75 (dd, J = 11.0, 4.4 Hz, 1H), 4.00 (dd, J = 8.0, 4.6 Hz, 1H), 4.90–5.03 (m, 1H), 7.29–7.47 (m, 5H). 13C NMR (126 MHz, CDCl3) δ 21.8, 29.4, 30.0, 30.9, 32.4, 33.8, 47.4, 59.7, 61.6, 66.5, 67.9, 120.5, 127.9, 128.5, 128.9, 140.1, 174.8. MS (LCMS) 343(MH+). Anal. calcd for C20H26N2O3 C 70.15, H 7.65, N 8.18. Found C 70.40, H 7.33, N 8.47.
1; UV). 1H NMR (500 MHz, CDCl3) δ 1.21 (d, J = 6.2 Hz, 6H), 1.96–2.25 (m, 8H), 2.32–2.41 (m, 1H), 2.42–2.54 (m, 1H), 2.67–2.54 (m, 1H), 3.60–3.70 (m, 1H), 3.75 (dd, J = 11.0, 4.4 Hz, 1H), 4.00 (dd, J = 8.0, 4.6 Hz, 1H), 4.90–5.03 (m, 1H), 7.29–7.47 (m, 5H). 13C NMR (126 MHz, CDCl3) δ 21.8, 29.4, 30.0, 30.9, 32.4, 33.8, 47.4, 59.7, 61.6, 66.5, 67.9, 120.5, 127.9, 128.5, 128.9, 140.1, 174.8. MS (LCMS) 343(MH+). Anal. calcd for C20H26N2O3 C 70.15, H 7.65, N 8.18. Found C 70.40, H 7.33, N 8.47.
Isopropyl (2R,4s,5R)-5-cyano-5-(((S)-2-hydroxy-1-phenyl ethyl)amino)spiro[3.3]heptane-2-carboxylate 16c
A mixture of 16c with benzyl alcohol (1.28 g, 77% of 16c by NMR, 2.87 mmol, 47% yield) was obtained analogously to 16a from 11b and used in the next step without any additional purification. Yellow oil. TLC: Rf = 0.38 (cyclohexane–EtOAc 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; UV). 1H NMR (500 MHz, CDCl3) δ 1.26 (dd, J = 6.2, 1.0 Hz, 6H), 1.49 (t, J = 9.8 Hz, 1H), 1.64–1.74 (m, 1H), 1.86–2.03 (m, 2H), 2.02–2.13 (m, 1H), 2.19–2.31 (m, 1H), 2.49 (dd, J = 11.6, 8.5 Hz, 1H), 2.57–2.92 (m, 3H), 2.94–3.05 (m, 1H), 3.61 (t, J = 10.0 Hz, 1H), 3.73 (dd, J = 10.9, 3.3 Hz, 1H), 4.07 (dd, J = 8.4, 3.6 Hz, 1H), 4.95–5.09 (m, 1H), 7.19–7.50 (m, 5H). 13C NMR (126 MHz, CDCl3) δ 21.8, 30.0, 31.0, 31.8, 34.3, 45.6, 60.1, 62.2, 67.1, 68.2, 77.2, 120.8, 127.9, 127.9, 128.4, 140.6, 175.0. MS (LCMS) 343(MH+).
1; UV). 1H NMR (500 MHz, CDCl3) δ 1.26 (dd, J = 6.2, 1.0 Hz, 6H), 1.49 (t, J = 9.8 Hz, 1H), 1.64–1.74 (m, 1H), 1.86–2.03 (m, 2H), 2.02–2.13 (m, 1H), 2.19–2.31 (m, 1H), 2.49 (dd, J = 11.6, 8.5 Hz, 1H), 2.57–2.92 (m, 3H), 2.94–3.05 (m, 1H), 3.61 (t, J = 10.0 Hz, 1H), 3.73 (dd, J = 10.9, 3.3 Hz, 1H), 4.07 (dd, J = 8.4, 3.6 Hz, 1H), 4.95–5.09 (m, 1H), 7.19–7.50 (m, 5H). 13C NMR (126 MHz, CDCl3) δ 21.8, 30.0, 31.0, 31.8, 34.3, 45.6, 60.1, 62.2, 67.1, 68.2, 77.2, 120.8, 127.9, 127.9, 128.4, 140.6, 175.0. MS (LCMS) 343(MH+).
Isopropyl (2S,4s,5S)-5-cyano-5-(((S)-2-hydroxy-1-phenyle thyl)amino)spiro[3.3]heptane-2-carboxylate (16d)
Compound 16d (0.5 g, 1.46 mmol, 24%) was obtained analogously to 16b from 11b. Yellow oil. TLC: Rf = 0.28 (cyclohexane–EtOAc 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; UV). 1H NMR (500 MHz, CDCl3) δ 1.22 (dd, J = 5.6, 3.8 Hz, 6H), 1.97–2.18 (m, 4H), 2.18–2.26 (m, 1H), 2.26–2.34 (m, 1H), 2.34–2.58 (m, 3H), 2.64 (dd, J = 11.5, 8.0 Hz, 1H), 2.89–3.00 (m, 1H), 3.59 (dd, J = 10.5, 8.3 Hz, 1H), 3.69 (dd, J = 10.8, 4.5 Hz, 1H), 3.90 (dd, J = 7.4, 4.7 Hz, 1H), 4.93–5.05 (m, 1H), 7.25–7.47 (m, 5H). 13C NMR (126 MHz, CDCl3) δ 21.8, 29.51, 29.57, 31.3, 31.9, 34.5, 46.0, 58.5, 60.8, 66.4, 68.2, 120.4, 127.5, 128.2, 128.8, 139.9, 174.8. MS (LCMS) 343 (MH+). Anal. calcd for C20H26N2O3 C 70.15, H 7.65, N 8.18. Found C 69.93, H 7.81, N 8.06.
1; UV). 1H NMR (500 MHz, CDCl3) δ 1.22 (dd, J = 5.6, 3.8 Hz, 6H), 1.97–2.18 (m, 4H), 2.18–2.26 (m, 1H), 2.26–2.34 (m, 1H), 2.34–2.58 (m, 3H), 2.64 (dd, J = 11.5, 8.0 Hz, 1H), 2.89–3.00 (m, 1H), 3.59 (dd, J = 10.5, 8.3 Hz, 1H), 3.69 (dd, J = 10.8, 4.5 Hz, 1H), 3.90 (dd, J = 7.4, 4.7 Hz, 1H), 4.93–5.05 (m, 1H), 7.25–7.47 (m, 5H). 13C NMR (126 MHz, CDCl3) δ 21.8, 29.51, 29.57, 31.3, 31.9, 34.5, 46.0, 58.5, 60.8, 66.4, 68.2, 120.4, 127.5, 128.2, 128.8, 139.9, 174.8. MS (LCMS) 343 (MH+). Anal. calcd for C20H26N2O3 C 70.15, H 7.65, N 8.18. Found C 69.93, H 7.81, N 8.06.
The ratio of 16c and 16d in the crude mixture was 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (NMR data).
2 (NMR data).
(1R,4r,6S)-1-Amino-spiro[3.3]heptane-1,6-dicarboxylic acid (1a), hydrochloride
Compound 16a (0.62 g, 84% by NMR, 1.53 mmol) was dissolved in CH2Cl2–MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (40 mL). The resulting solution was cooled to 0 °C, and Pb(OAc)4 (1.21 g, 2.73 mmol) was added quickly. After 10 min at 0 °C, saturated aq. NaHCO3 (50 mL) was added. The aqueous layer was extracted with CH2Cl2 (20 mL). The organic extract was filtered and the precipitate was washed with CH2Cl2 (15 mL). The combined organic phases were dried over Na2SO4 and evaporated. The residue was dissolved in CH2Cl2 (20 mL) and saturated with gaseous HCl for 20 min at 0 °C. MeOH (5 mL) was added, and the resulting mixture was saturated with HCl for another 20 min at 0 °C. The reaction mixture was stirred overnight at ambient temperature, and then evaporated. The residue was dissolved in 6 N aq. HCl (30 mL) and refluxed for 2 h, then washed with Et2O (3 × 15 mL), evaporated, dissolved in water (15 mL). This solution was made alkaline with an excess of NaOH and evaporated to dryness. The residue was dissolved in 6 N aq. HCl and evaporated to dryness again. The resulting residue was dissolved in dry ethanol (15 mL), and the precipitate was filtered off. The filtrate was concentrated in vacuo and the resulting solid was recrystallized from 2-propanol to give hydrochloride of 1a (0.28 g, 1.19 mmol, 78%). White solid. Mp 213–215 °C (dec). [α]20D = −13.6 (c 0.5, H2O). 1H NMR (500 MHz, D2O) δ 2.14–2.46 (m, 6H), 2.50–2.60 (m, 1H), 2.68 (t, J = 11.1 Hz, 1H), 3.02–3.12 (m, 1H). 13C NMR (126 MHz, D2O) δ 24.5, 30.7, 32.0, 32.2, 33.9, 44.7, 62.2, 172.4, 179.7. MS (LCMS) 200 (MH+ − Cl−). Anal. calcd for C9H14ClNO4 C 45.87, H 5.99, Cl 5.04, N 5.94. Found C 45.49, H 6.31, Cl 4.77, N 5.90.
1) (40 mL). The resulting solution was cooled to 0 °C, and Pb(OAc)4 (1.21 g, 2.73 mmol) was added quickly. After 10 min at 0 °C, saturated aq. NaHCO3 (50 mL) was added. The aqueous layer was extracted with CH2Cl2 (20 mL). The organic extract was filtered and the precipitate was washed with CH2Cl2 (15 mL). The combined organic phases were dried over Na2SO4 and evaporated. The residue was dissolved in CH2Cl2 (20 mL) and saturated with gaseous HCl for 20 min at 0 °C. MeOH (5 mL) was added, and the resulting mixture was saturated with HCl for another 20 min at 0 °C. The reaction mixture was stirred overnight at ambient temperature, and then evaporated. The residue was dissolved in 6 N aq. HCl (30 mL) and refluxed for 2 h, then washed with Et2O (3 × 15 mL), evaporated, dissolved in water (15 mL). This solution was made alkaline with an excess of NaOH and evaporated to dryness. The residue was dissolved in 6 N aq. HCl and evaporated to dryness again. The resulting residue was dissolved in dry ethanol (15 mL), and the precipitate was filtered off. The filtrate was concentrated in vacuo and the resulting solid was recrystallized from 2-propanol to give hydrochloride of 1a (0.28 g, 1.19 mmol, 78%). White solid. Mp 213–215 °C (dec). [α]20D = −13.6 (c 0.5, H2O). 1H NMR (500 MHz, D2O) δ 2.14–2.46 (m, 6H), 2.50–2.60 (m, 1H), 2.68 (t, J = 11.1 Hz, 1H), 3.02–3.12 (m, 1H). 13C NMR (126 MHz, D2O) δ 24.5, 30.7, 32.0, 32.2, 33.9, 44.7, 62.2, 172.4, 179.7. MS (LCMS) 200 (MH+ − Cl−). Anal. calcd for C9H14ClNO4 C 45.87, H 5.99, Cl 5.04, N 5.94. Found C 45.49, H 6.31, Cl 4.77, N 5.90.
(1S,4r,6R)-1-Amino-spiro[3.3]heptane-1,6-dicarboxylic acid (1b), hydrochloride
Compound 1b (0.14 g, 0.59 mmol, 81%) was obtained from 16b analogously to 1a. White solid. Mp 212–214 °C (dec). [α]20D = +10.0 (c 0.5, H2O). 1H NMR (500 MHz, D2O) δ 2.14–2.46 (m, 6H), 2.50–2.60 (m, 1H), 2.68 (t, J = 11.1 Hz, 1H), 3.02–3.12 (m, 1H). 13C NMR (126 MHz, D2O) δ 24.6, 30.7, 32.0, 32.3, 34.0, 44.8, 62.3, 172.4, 179.7. MS (LCMS) 200 (MH+ − Cl−). Anal. calcd for C9H14ClNO4 C 45.87, H 5.99, Cl 5.04, N 5.94. Found C 45.60, H 5.91, Cl 5.26, N 5.71.(1R,4s,6R)-1-Amino-spiro[3.3]heptane-1,6-dicarboxylic acid (1c), hydrochloride
Compound 1c (0.28 g, 1.19 mmol, 83%) was obtained analogously to 1a from 16c (0.64 g, 77% by NMR, 1.44 mmol). White solid. Mp 224–226 °C (dec). [α]20D = +13.2 (c 0.5, H2O). 1H NMR (500 MHz, D2O) δ 2.16–2.36 (m, 5H), 2.38–2.51 (m, 2H), 2.53–2.61 (m, 1H), 3.13–3.26 (m, 1H). 13C NMR (126 MHz, D2O) δ 24.7, 29.6, 30.8, 33.1, 34.8, 42.6, 61.9, 172.6, 179.1. MS (LCMS) 200 (MH+ − Cl−). Anal. calcd for C9H14ClNO4 C 45.87, H 5.99, Cl 5.04, N 5.94. Found C 45.66, H 6.05, Cl 5.04, N 5.52.(1S,4s,6S)-1-Amino-spiro[3.3]heptane-1,6-dicarboxylic acid (1d), hydrochloride
Compound 1d (0.12 g, 0.51 mmol, 70%) was obtained analogously to 1a from 16d (0.25 g, 0.73 mmol). White solid. Mp 224–226 °C (dec). [α]20D = −17.2 (c 0.5, H2O). 1H NMR (500 MHz, D2O) δ 2.16–2.36 (m, 5H), 2.38–2.51 (m, 2H), 2.53–2.61 (m, 1H), 3.13–3.26 (m, 1H). 13C NMR (126 MHz, D2O) δ 24.7, 29.6, 30.8, 33.1, 34.8, 42.6, 61.9, 172.6, 179.1. MS (LCMS) 200 (MH+ − Cl−). Anal. calcd for C9H14ClNO4 C 45.87, H 5.99, Cl 5.04, N 5.94. Found C 46.03, H 6.06, Cl 5.17, N 6.31.Methyl (2S,4r,5R,7S)-10-oxo-7-phenyl-9-oxa-6-azadispiro[3.0.5.2]dodecane-2-carboxylate (18a)
16a (0.62 g, 84% purity by NMR, 1.53 mmol) was dissolved in CH2Cl2 (20 mL) and saturated with gaseous HCl for 20 min at 0 °C. MeOH (5 mL) was added, and the resulting mixture was saturated with HCl for another 20 min at 0 °C. The reaction mixture was stirred overnight at ambient temperature, and then evaporated. The residue was treated with CH2Cl2 (20 mL). The resulting solution was washed with saturated aq. NaHCO3 and dried over Na2SO4. Then the solvent was evaporated and the crude product was purified form by column chromatography (cyclohexane–EtOAc (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as an eluent). The yield of 18a was 0.24 g (0.76 mmol, 50%). White solid. Mp 130–131 °C. TLC: Rf = 0.50 (cyclohexane–EtOAc 2
1) as an eluent). The yield of 18a was 0.24 g (0.76 mmol, 50%). White solid. Mp 130–131 °C. TLC: Rf = 0.50 (cyclohexane–EtOAc 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; UV). [α]20D = −3.11 (c 0.55, CHCl3). 1H NMR (500 MHz, CDCl3) δ 1.64–1.75 (m, 1H), 1.83–1.99 (m, 2H), 2.16 (dd, J = 11.3, 5.8 Hz, 1H), 2.28–2.37 (m, 3H), 2.68–2.78 (m, 1H), 2.91–3.05 (m, 2H), 3.69 (s, 3H), 4.14 (t, J = 10.7 Hz, 1H), 4.26 (dd, J = 10.6, 3.2 Hz, 1H), 4.31 (dd, J = 10.8, 3.1 Hz, 1H), 7.30–7.43 (m, 3H), 7.46 (d, J = 6.9 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 29.7, 31.7, 32.8, 34.3, 35.6, 49.2, 51.9, 54.3, 65.0, 74.2, 127.3, 128.8, 129.0, 138.1, 171.1, 176.0. MS (LCMS) 316 (MH+). Anal. Calcd for C18H21NO4 C 68.55, H 6.71, N 4.44. Found C 68.37, H 6.93, N 4.26.
1; UV). [α]20D = −3.11 (c 0.55, CHCl3). 1H NMR (500 MHz, CDCl3) δ 1.64–1.75 (m, 1H), 1.83–1.99 (m, 2H), 2.16 (dd, J = 11.3, 5.8 Hz, 1H), 2.28–2.37 (m, 3H), 2.68–2.78 (m, 1H), 2.91–3.05 (m, 2H), 3.69 (s, 3H), 4.14 (t, J = 10.7 Hz, 1H), 4.26 (dd, J = 10.6, 3.2 Hz, 1H), 4.31 (dd, J = 10.8, 3.1 Hz, 1H), 7.30–7.43 (m, 3H), 7.46 (d, J = 6.9 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 29.7, 31.7, 32.8, 34.3, 35.6, 49.2, 51.9, 54.3, 65.0, 74.2, 127.3, 128.8, 129.0, 138.1, 171.1, 176.0. MS (LCMS) 316 (MH+). Anal. Calcd for C18H21NO4 C 68.55, H 6.71, N 4.44. Found C 68.37, H 6.93, N 4.26.
Methyl (2R,4r,5S,7S)-10-oxo-7-phenyl-9-oxa-6-azadispiro[3.0.5.2]dodecane-2-carboxylate (18b)
Compound 18b (0.14 g, 0.44 mmol, 61%) was obtained from 16b (0.25 g, 0.73 mmol) analogously to 18a. White solid. Mp 142–143 °C. TLC: Rf = 0.41 (cyclohexane–EtOAc 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; UV). [α]20D = +12.89 (c 0.18, CHCl3). 1H NMR (500 MHz, CDCl3) δ 1.62–1.75 (m, 1H), 1.88 (t, J = 9.6 Hz, 1H), 2.13–2.23 (m, 1H), 2.25–2.38 (m, 3H), 2.39–2.50 (m, 1H), 2.52–2.69 (m, 2H), 3.03–3.17 (m, 1H), 3.70 (s, 3H), 4.21–4.43 (m, 3H), 7.30–7.51 (m, 5H). 13C NMR (126 MHz, CDCl3) δ 30.2, 30.5, 31.6, 32.3, 35.4, 49.8, 52.1, 53.9, 65.8, 74.7, 127.2, 129.1, 129.2, 137.4, 170.6, 175.8. MS (LCMS) 316 (MH+). Anal. calcd for C18H21NO4 C 68.55, H 6.71, N 4.44. Found C 68.70, H 6.52, N 4.29.
1; UV). [α]20D = +12.89 (c 0.18, CHCl3). 1H NMR (500 MHz, CDCl3) δ 1.62–1.75 (m, 1H), 1.88 (t, J = 9.6 Hz, 1H), 2.13–2.23 (m, 1H), 2.25–2.38 (m, 3H), 2.39–2.50 (m, 1H), 2.52–2.69 (m, 2H), 3.03–3.17 (m, 1H), 3.70 (s, 3H), 4.21–4.43 (m, 3H), 7.30–7.51 (m, 5H). 13C NMR (126 MHz, CDCl3) δ 30.2, 30.5, 31.6, 32.3, 35.4, 49.8, 52.1, 53.9, 65.8, 74.7, 127.2, 129.1, 129.2, 137.4, 170.6, 175.8. MS (LCMS) 316 (MH+). Anal. calcd for C18H21NO4 C 68.55, H 6.71, N 4.44. Found C 68.70, H 6.52, N 4.29.
Methyl (2R,4s,5R,7S)-10-oxo-7-phenyl-9-oxa-6-azadispiro[3.0.55.24]dodecane-2-carboxylate (18c)
Compound 18c (0.26 g, 0.82 mmol, 57%) was obtained analogously to 18a from 16c (0.64 g, 77% purity by NMR, 1.44 mmol). White solid. Mp 111–111 °C. TLC: Rf = 0.49 (cyclohexane–EtOAc 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; UV). [α]20D = −30.28 (c 0.455, CHCl3). 1H NMR (500 MHz, CDCl3) δ 1.71–1.85 (m, 2H), 1.95–2.06 (m, 2H), 2.22–2.33 (m, 2H), 2.34–2.43 (m, 1H), 2.73–2.81 (m, 1H), 2.89 (t, J = 10.0 Hz, 1H), 3.00–3.10 (m, 1H), 3.69 (s, 3H), 4.20–4.31 (m, 3H), 7.31–7.42 (m, 3H), 7.48 (d, J = 7.4 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 29.5, 31.3, 32.6, 33.9, 34.8, 47.3, 51.9, 54.2, 64.5, 74.3, 127.4, 128.8, 129.0, 138.2, 170.9, 175.7. MS (LCMS) 316 (MH+). Anal. calcd for C18H21NO4 C 68.55, H 6.71, N 4.44. Found C 68.42, H 6.58, N 4.14.
1; UV). [α]20D = −30.28 (c 0.455, CHCl3). 1H NMR (500 MHz, CDCl3) δ 1.71–1.85 (m, 2H), 1.95–2.06 (m, 2H), 2.22–2.33 (m, 2H), 2.34–2.43 (m, 1H), 2.73–2.81 (m, 1H), 2.89 (t, J = 10.0 Hz, 1H), 3.00–3.10 (m, 1H), 3.69 (s, 3H), 4.20–4.31 (m, 3H), 7.31–7.42 (m, 3H), 7.48 (d, J = 7.4 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 29.5, 31.3, 32.6, 33.9, 34.8, 47.3, 51.9, 54.2, 64.5, 74.3, 127.4, 128.8, 129.0, 138.2, 170.9, 175.7. MS (LCMS) 316 (MH+). Anal. calcd for C18H21NO4 C 68.55, H 6.71, N 4.44. Found C 68.42, H 6.58, N 4.14.
Methyl (2S,4s,5S,7S)-10-oxo-7-phenyl-9-oxa-6-azadispiro[3.0.55.24]dodecane-2-carboxylate (18d)
Compound 18d (0.12 g, 0.38 mmol, 52%) was obtained analogously to 18b from 16d (0.25 g, 0.73 mmol). White solid. Mp 79–80 °C. TLC: Rf = 0.40 (cyclohexane–EtOAc 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; UV). [α]20D = +32.2 (c 0.25, CHCl3). 1H NMR (500 MHz, CDCl3) δ 1.66–1.77 (m, 1H), 1.89 (t, J = 9.9 Hz, 1H), 1.95–2.06 (m, 1H), 2.16–2.45 (m, 4H), 2.54–2.70 (m, 2H), 3.18–3.04 (m, 1H), 3.72 (s, 3H), 4.25–4.39 (m, 2H), 4.51–4.64 (m, 1H), 7.30–7.44 (m, 3H), 7.48 (d, J = 5.9 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 30.0, 30.1, 30.2, 32.4, 36.7, 48.4, 52.1, 53.4, 65.4, 74.8, 127.5, 128.9, 129.1, 137.6, 170.7, 176.0. MS (LCMS) 316 (MH+). Anal. calcd for C18H21NO4 C 68.55, H 6.71, N 4.44. Found C 68.90, H 6.41, N 4.63.
1; UV). [α]20D = +32.2 (c 0.25, CHCl3). 1H NMR (500 MHz, CDCl3) δ 1.66–1.77 (m, 1H), 1.89 (t, J = 9.9 Hz, 1H), 1.95–2.06 (m, 1H), 2.16–2.45 (m, 4H), 2.54–2.70 (m, 2H), 3.18–3.04 (m, 1H), 3.72 (s, 3H), 4.25–4.39 (m, 2H), 4.51–4.64 (m, 1H), 7.30–7.44 (m, 3H), 7.48 (d, J = 5.9 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 30.0, 30.1, 30.2, 32.4, 36.7, 48.4, 52.1, 53.4, 65.4, 74.8, 127.5, 128.9, 129.1, 137.6, 170.7, 176.0. MS (LCMS) 316 (MH+). Anal. calcd for C18H21NO4 C 68.55, H 6.71, N 4.44. Found C 68.90, H 6.41, N 4.63.
Dimethyl (1R,4s,6R)-1-(((S)-2-hydroxy-1-phenylethyl)amino)spiro[3.3]heptane-1,6-dicarboxylate (19c)
Compound 19c (0.11 g, 0.32 mmol, 22%) was obtained from 16c during chromatographic purification of 18c. White solid. Mp 68–69 °C. TLC: Rf = 0.36 (cyclohexane–EtOAc 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; UV). [α]20D = +25.2 (c 0.46, CHCl3). 1H NMR (500 MHz, CDCl3) δ 1.39–1.52 (m, 1H), 1.90–2.07 (m, 4H), 2.07–2.21 (m, 2H), 2.64–2.74 (m, 1H), 2.89–3.02 (m, 1H), 3.45–3.57 (m, 1H), 3.57–3.65 (m, 2H), 3.69 (s, 3H), 3.77 (s, 3H), 7.20–7.41 (m, 5H). 13C NMR (126 MHz, CDCl3) δ 25.1, 30.5, 31.6, 33.1, 34.8, 45.5, 51.9, 52.1, 61.7, 67.6, 67.7, 127.3, 127.5, 128.5, 142.6, 175.4, 175.8. MS (LCMS) 348 (MH+). Anal. calcd for C19H25NO5 C 65.69, H 7.25, N 4.03. Found C 65.47, H 7.04, N 3.85.
1; UV). [α]20D = +25.2 (c 0.46, CHCl3). 1H NMR (500 MHz, CDCl3) δ 1.39–1.52 (m, 1H), 1.90–2.07 (m, 4H), 2.07–2.21 (m, 2H), 2.64–2.74 (m, 1H), 2.89–3.02 (m, 1H), 3.45–3.57 (m, 1H), 3.57–3.65 (m, 2H), 3.69 (s, 3H), 3.77 (s, 3H), 7.20–7.41 (m, 5H). 13C NMR (126 MHz, CDCl3) δ 25.1, 30.5, 31.6, 33.1, 34.8, 45.5, 51.9, 52.1, 61.7, 67.6, 67.7, 127.3, 127.5, 128.5, 142.6, 175.4, 175.8. MS (LCMS) 348 (MH+). Anal. calcd for C19H25NO5 C 65.69, H 7.25, N 4.03. Found C 65.47, H 7.04, N 3.85.
X-ray diffraction studies
X-Ray diffraction studies were performed on an automatic “Xcalibur 3” diffractometer (graphite monochromated MoKα radiation, CCD-detector, ω-scanning). The crystals were obtained by slow evaporation of the solutions in MeCN (13) or cyclohexane–EtOAc (18a, 18b and 18c). The structure was solved by direct method using SHELXTL package.24 Positions of hydrogen atoms were located from electron density difference maps and refined using riding model with Uiso = nUeq (n = 1.5 for methyl groups and 1.2 for other hydrogen atoms). In the case of 18a–c, hydrogen atoms at N(1) were refined using isotropic model. The crystallographic data and experimental parameters are listed in ESI, Table S1.† Final atomic coordinates, geometrical parameters and crystallographic data have been deposited with the Cambridge Crystallographic Data Centre, 11 Union Road, Cambridge, CB2 1EZ, UK (fax: +44 1223 336033; e-mail: E-mail: deposit@ccdc.cam.ac.uk). The deposition numbers are given in Table S1.†Acknowledgements
The authors thank Dr Valeriya Makhankova for her invaluable help with manuscript preparation, and Mr Vitaliy Polovinko for NMR experiments.Notes and references
- G. A. Pinna, G. Murineddu, M. M. Curzu, S. Villa, P. Vianello, P. A. Borea, S. Gessi, L. Toma, D. Colombo and G. Cignarella, Farmaco, 2000, 55, 553 CrossRef CAS.
- M. C. Desai and S. L. Lefkowitz, Tetrahedron Lett., 1994, 35, 4701–4704 CrossRef CAS.
- G. W. Bemis and M. A. Murcko, J. Med. Chem., 1996, 39, 2887–2893 CrossRef CAS PubMed.
- J. Wang and T. Hou, J. Chem. Inf. Model., 2010, 50, 55–67 CrossRef CAS PubMed.
- O. O. Grygorenko, D. S. Radchenko, D. M. Volochnyuk, A. A. Tolmachev and I. V. Komarov, Chem. Rev., 2011, 111, 5506–5568 CrossRef CAS PubMed.
- A. de Meijere, K. Ernst, B. Zuck, M. Brandl, S. I. Kozhushkov, M. Tamm, D. S. Yufit, J. A. K. Howard and T. Labahn, Eur. J. Org. Chem., 1999, 3105–3115 CrossRef CAS.
- (a) S. Kotha, A. C. Deb, K. Lahiri and E. Manivannan, Synthesis, 2009, 165–193 CrossRef CAS PubMed; (b) S. Rosenberg and R. Leino, Synthesis, 2009, 2651–2673 CAS.
- (a) W. Lin, A. Gupta, K. H. Kim, D. Mendel and M. J. Miller, Org. Lett., 2009, 11, 449–452 CrossRef CAS PubMed; (b) Z. Guo, P. Orth, S.-C. Wong, B. J. Lavey, N.-Y. Shih, X. Niu, D. J. Lundell, V. Madison and J. A. Kozlowski, Bioorg. Med. Chem. Lett., 2009, 19, 54–57 CrossRef CAS PubMed; (c) L. Lomlim, J. Einsiedel, F. W. Heinemann, K. Meyer and P. Gmeiner, J. Org. Chem., 2008, 73, 3608–3611 CrossRef CAS PubMed; (d) J. A. Pfefferkorn and C. Choi, Tetrahedron Lett., 2008, 49, 4372–4373 CrossRef CAS PubMed; (e) A. Pasternak, S. D. Goble, G. A. Doss, N. N. Tsou, G. Butora, P. P. Vicario, J. M. Ayala, M. Struthers, J. A. DeMartino, S. G. Mills and L. Yang, Bioorg. Med. Chem. Lett., 2008, 18, 1374–1377 CrossRef CAS PubMed; (f) A. F. Gasiecki, J. L. Cross, R. F. Henry, V. Gracias and S. W. Djuric, Tetrahedron Lett., 2008, 49, 6286–6288 CrossRef CAS PubMed; (g) E. Prusov and M. E. Maier, Tetrahedron, 2007, 63, 10486 CrossRef CAS PubMed; (h) M. Wurdemann and J. Christoffers, Org. Biomol. Chem., 2010, 8, 1894–1898 RSC.
- J. Fröhlich, F. Sauter and K. Blasl, Heterocycles, 1994, 37, 1879 CrossRef.
- J. A. Burkhard, B. Wagner, H. Fischer, F. Schuler, K. Müller and E. M. Carreira, Angew. Chem., Int. Ed., 2010, 49, 3524 CrossRef CAS PubMed.
- D. S. Radchenko, O. O. Grygorenko and I. V. Komarov, Tetrahedron: Asymmetry, 2008, 19, 2924–2930 CrossRef CAS PubMed.
- F. Tellier, F. Acher, I. Brabet, J.-P. Pin and R. Azerad, Bioorg. Med. Chem., 1998, 6, 195–208 CrossRef CAS.
- A. P. Kozikowski, D. Steensma, G. L. Araldi, W. Tückmantel, S. Wang, S. Pshenichkin, E. Surina and J. T. Wroblewski, J. Med. Chem., 1998, 41, 1641–1650 CrossRef CAS PubMed.
- J. A. Monn, M. J. Valli, S. M. Massey, R. A. Wright, C. R. Salhoff, B. G. Johnson, T. Howe, C. A. Alt, G. A. Rhodes, R. L. Robey, K. R. Griffey, J. P. Tizzano, M. J. Kallman, D. R. Helton and D. D. Schoepp, J. Med. Chem., 1997, 40, 528–537 CrossRef CAS PubMed.
- H. Bräuner-Osborne, J. Egebjerg, E. Ø. Nielsen, U. Madsen and P. Krogsgaard-Larsen, J. Med. Chem., 2000, 43, 2609–2645 CrossRef.
- B. M. Trost and M. J. Bogdanovich, J. Am. Chem. Soc., 1973, 95, 5321–5334 CrossRef CAS.
- H. Nemoto, M. Shiraki and K. Fukumoto, J. Org. Chem., 1996, 61, 1347–1353 CrossRef CAS.
- D. S. Radchenko, O. M. Michurin, O. O. Grygorenko, K. Scheinpflug, M. Dathe and I. V. Komarov, Tetrahedron, 2013, 69, 505–511 CrossRef CAS PubMed.
- C. Guérot, B. H. Tschitschanov, H. Knust and E. M. Carreira, Org. Lett., 2001, 13, 780–783 CrossRef PubMed.
- M. Hamaguchi, Y. Iyama, E. Mochizuki and T. Oshima, Tetrahedron Lett., 2005, 46, 8949–8952 CrossRef CAS PubMed.
- A. V. Malkov, F. Friscourt, M. Bell, M. E. Swarbrick and P. Kočovský, J. Org. Chem., 2008, 73, 3996–4003 CrossRef CAS PubMed.
- A. Maercker and V. E. E. Daub, Tetrahedron, 1994, 50, 2439–2458 CrossRef CAS.
- D. S. Radchenko, S. O. Pavlenko, O. O. Grygorenko, D. M. Volochnyuk, S. V. Shishkina, O. V. Shishkin and I. V. Komarov, J. Org. Chem., 2010, 75, 5941–5952 CrossRef CAS PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Including copies of NMR spectra. CCDC 962474, 962475, 962476, 962477. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3ra47725h |
| This journal is © The Royal Society of Chemistry 2014 |