Pyrrolidine and piperidine based chiral spiro and fused scaffolds via build/couple/pair approach†
Rajinikanth Mamidalab,
V. Surendra Babu Damerlab,
Rambabu Gundlab,
M. Thirumala Charyc,
Y. L. N. Murthyd and
Subhabrata Sen*a
aDepartment of Chemistry, Shiv Nadar University, Dadri, UP, India. E-mail: subhabrata.sen@snu.edu.in
bGVK Bioscience, 28A IDA Nacharam, Hyderabad, AP, India
cJNTU-H College, Nachupally, Karimnagar, AP, India
dAndhra University, Vishakhapatnam, AP, India
First published on 3rd February 2014
Abstract
A versatile stereoselective diversity oriented synthetic pathway to the possible spiro and fused diverse heterocyclic small molecules is described. The strategy involved the “build–couple–pair” approach involving an SNAr, Michael addition and Mannich reaction on chiral acyl bicyclic lactams 2a/b, followed by a cyclization onto the inbuilt scaffold electrophile, thereby leading to asymmetric fused and spirocyclic nitrogen heterocycles. A “post-pair” phase has been incorporated to generate more polar compounds. We used Principal Component Analysis (PCA) and polar moment of inertia to evaluate the shape-space diversity of our scaffolds with respect to a commercial database and observed extraordinary diversity within the scaffold network. We further calculated the polar surface area (PSA) of our molecules which is an indicator for drug cell permeability.
Introduction
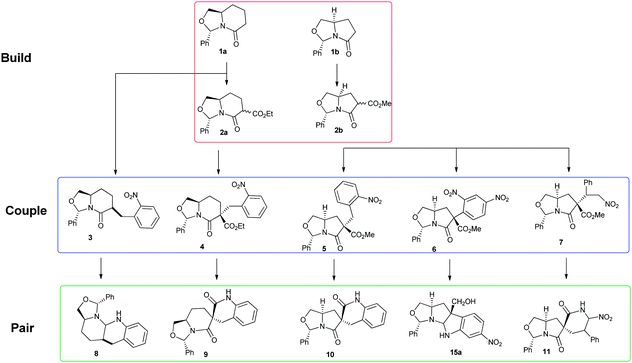
Interactive abilities of small molecules with biomacromolecules like DNA and RNA are a critical factor in analysing biological processes.1 They form the fundamental platform of modern pharmaceutical science. These superficial three-dimensional interactions have inspired diversity oriented synthesis (DOS), to design molecules that mimic them.2 Due to the unbiased nature of DOS libraries they exhibit a varied range of physicochemical and biological properties, and hence can be used for screening to identify novel compounds.3In recent years, build/couple/pair (B/C/P) algorithm of DOS has evolved as an extremely successful technique to generate architecturally and stereochemically diverse scaffolds.4 We envisioned a collection of diverse shaped chiral spiro- and fused scaffolds via the B/C/P strategy. Scheme 1 illustrates our strategy where the “build” phase generates stereochemically diverse chiral bicyclic lactams 1a and 1b as building blocks. In the “couple” phase, the building blocks are equipped with orthogonal functionalities via enolisation–substitution and final “pairing” involved intramolecular cyclization onto the inbuilt scaffold electrophile between the embedded functionalities to generate higher order, more complicated structures.
The ready availability of these acyl bicyclic lactam precursors from chiral aminols is key to the success of the B/C/P strategy described in this article. The bicyclic lactams 1a and 1b can be generated easily from the condensation of (i) R-(6-hydroxymethy)-piperidinone and (ii) R-pyroglutaminol with benzaldehyde.5 Followed by acylation with α-chloroformate to afford 2a and 2b.6,7 These chiral acyl bicyclic lactams, by virtue of their susceptability towards enolate mediated nucleophilic reactions at the carbon α- to the amide bond are extremely popular intermediates to synthesize various nitrogen and oxygen heterocycles.8 In our case we coupled them (2a/2b) with appropriate reagents via an array of efficient intermolecular reactions such as (i) Michael addition; (ii) aromatic nucleophilic substitution (SNAr); and (iii) Mannich reaction, to generate more densely functionalized scaffolds. Finally, functional group (FG) pairing afforded skeletally and stereochemically diverse scaffolds containing, disparate physicochemical properties (Scheme 1).
We wanted our scaffolds to abide by the “rule of three” as close as possible (M < 300; HBD ≤ 3 and HBA ≤ 3; c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 3; number of rotatable bonds ≤ 3; polar surface area = 60 Å2). These are acceptable med-chem attributes for creating fragment based libraries.9 Chiral synthesis of only cyclotryptamines (15a/15) have been reported earlier.10 However, to the best of our knowledge, this is the first B/C/P based DOS methodology that provides access to all of these structurally diverse molecular scaffolds through common intermediates (1a and 1b).
P = 3; number of rotatable bonds ≤ 3; polar surface area = 60 Å2). These are acceptable med-chem attributes for creating fragment based libraries.9 Chiral synthesis of only cyclotryptamines (15a/15) have been reported earlier.10 However, to the best of our knowledge, this is the first B/C/P based DOS methodology that provides access to all of these structurally diverse molecular scaffolds through common intermediates (1a and 1b).
Results and discussion
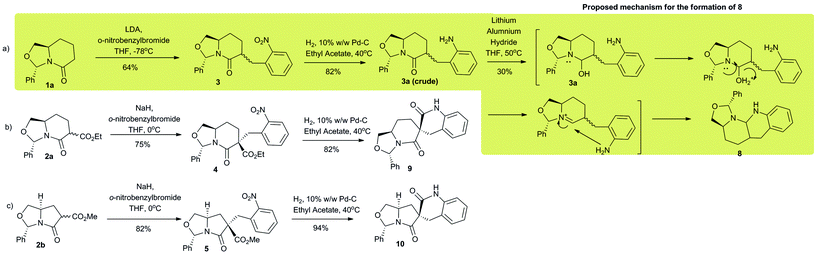
The coupling phase of the strategy initiated with simple alkylation of 1a, 2a and 2b with o-nitroarylbenzyl bromide to generate the corresponding alkylated intermediates 3, 4 and 5 respectively (Scheme 2). Exo-3 which is obtained from o-nitrobenzylation of 1a, by flash column chromatography of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 exo–endo mixture was reduced to amine under hydrogenation condition (10% w/w Pd–C, H2 at atm. pr.) (Scheme 2). The crude amine was treated with lithium aluminium hydride (LAH) at 50° and was transformed into a tetrafused carbocycle 8. We envisioned this conversion via an acyliminium cation mediated pathway as depicted in the Scheme 2. For representative purpose we chose to move ahead with the exo-diastereomer of 3 to generate 8, as shown in Scheme 2.
1 exo–endo mixture was reduced to amine under hydrogenation condition (10% w/w Pd–C, H2 at atm. pr.) (Scheme 2). The crude amine was treated with lithium aluminium hydride (LAH) at 50° and was transformed into a tetrafused carbocycle 8. We envisioned this conversion via an acyliminium cation mediated pathway as depicted in the Scheme 2. For representative purpose we chose to move ahead with the exo-diastereomer of 3 to generate 8, as shown in Scheme 2.
Intermediates 4 and 5 were generated with high diastereoselectivity (diastereomeric ratio ∼98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). The relative configuration was established via NOE (refer ESI†). Hydrogenation of the aryl nitro groups, afforded the amines, which cyclize in situ with the built in adjacent electrophilic carbonyl carbon of the ester to generate the spiro-compounds 9 and 10 with no loss of chirality.
2). The relative configuration was established via NOE (refer ESI†). Hydrogenation of the aryl nitro groups, afforded the amines, which cyclize in situ with the built in adjacent electrophilic carbonyl carbon of the ester to generate the spiro-compounds 9 and 10 with no loss of chirality.
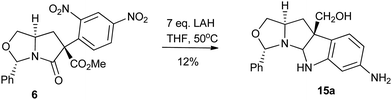
For the synthesis of cyclotryptamine 15a we used arylated intermediate 6, which was already reported in one of our early publications.6 There we have also reported the relative configuration of 6 by single crystal X-ray analysis. We used 6 for the synthesis of spiroxoindolinones (via Pd–C mediated hydrogenation of the nitro group on the benzene ring that led to an in situ lactamization). Such hydrogenation was not an option for our present effort. We envisioned an approach that would initiate with the ester reduction followed by the reduction of the nitro group and finally acylimine cation mediated cyclization of 6. Careful exploration of reagents (viz. diisobutylaluminium hydride [DIBAL-H, no conversion], Red-Al [no conversion], super hydride [no conversion], borane–DMS [no conversion]) prompted us to identify LAH as the most suitable reducing agent which facilitated this transformation to 15a, all be it in extremely poor yield of 12% and 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr (Scheme 3).
5 dr (Scheme 3).
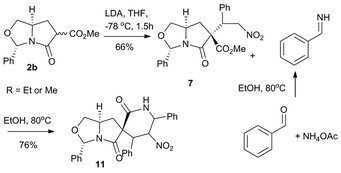
To access 7, chiral acyl bicyclic lactam 2b was treated with NaH at 0 °C and the resulting enolate was reacted with nitrostyrene. It was generated in decent yield (∼66%) and high diastereoselectivity (∼95%). Finally using Mültstadt's nitro-Mannich lactamization cascade we could transform 7 into the 5-5-6 spiro-scaffold 11.11 In this sequence 7 is reacted with benzyl imine generated in situ by treating benzaldehyde with ammonium acetate in ethanol at 80 °C to afford the desired product 11 as a single diastereomer (∼99%) with 76% yield. The relative configuration of the compound is derived from NOE/1H NMR (refer ESI†) data and is depicted in the scheme below (Scheme 4).
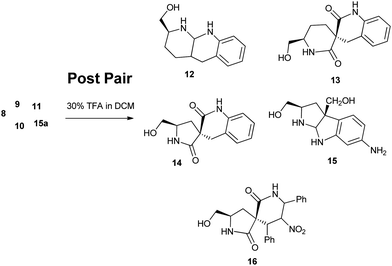
Along with shape, a DOS library should be prudent to possess physical properties for screening and promote follow-up chemistry. Transformational handle such as an alcoholic group is deliberately incorporated in the final compounds (12–16). In addition to structural and stereochemical diversity, this is a third diversity attribute in our library imbibed via a “postpairing phase.” This polar, alcohol group augment the solubility of these compounds, and will also serve as hydrogen-bonding donors (HBD) and acceptors (HBA), thereby improving binding affinity against bio-macromolecular targets. Last but not the least, this functional group was envisioned to facilitate scaffold proliferation (growing and transformation) into drug-like compounds.
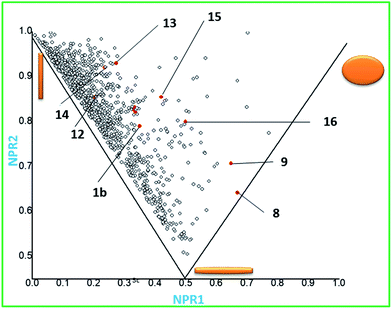
Molecular architecture and biological activity go hand in hand.12 Hence, screening libraries with higher degree of built-in molecular-shape diversity transcribe into a greater probability of discovering compounds with biological activity. In order to assess the overall diversity level of our library we used a computational method based on the normalized principal moment of inertia (PMI) formalism of Sauer and Schwartz.13 The PMI calculations involved aligning each molecule to the principal moment axes in SYBYL and the normalized PMI values were calculated by using in-house software. The commercial compounds were the FDA-approved drugs curated from the GOSTAR (GVK Bio Online Structure Activity Relationship)™ database.14 These compounds along with our scaffolds were imported into DS 3.1 and converted into 3D structures. Energy minimization was performed for all of these 3D structures to identify global/local minima by applying the CHARMm force field. The resulting 3D structures were used to calculate the three principal moments of inertia; the PMI values are sorted in ascending magnitude, that is, I1, I2, and I3. Normalized PMI ratios (NPR) were calculated by dividing the two smaller PMI values (I1 and I2) by the largest PMI value (I3) and generated two characteristic values for each compound (I1/I3 and I2/I3). These values were plotted against each other and the resulting graph formed an isosceles triangle that was defined by its three corners, wherein the vector [I1/I3, I2/I3] was equal to [1, 1], [0.5, 0.5], and [0, 1] (Fig. 1c) The three corners of the isosceles triangle of the PMI plot are dominated by rods, spheres, and discs, respectively (the shapes are representations of the overall shape of the constituent molecules). FDA approved drugs tend to occupy evenly over the shapes between rods and discs. Fig. 1 shows the coverage and distribution of majority of our scaffolds (1b, 8–9 and 12–16).
Our collection of scaffolds can be classified into two types of architectures, spiro- (9–11 and 13, 14 and 16), and fused (8, 12, 15a and 15). The figure above illustrates a wide distribution of our scaffolds across the PMI plot. It is noteworthy that majority of the scaffolds (1b and 12–14) from the post pair phase overlay on the same space as the FDA approved drugs, thereby emphasizing their drug like attributes. However there are quite a few outliers viz. 8, 9, 15 and 16. Interestingly these outliers consisted of both the spiro- and fused compounds. The advent of these outliers indicates a novel therapeutic domain that is yet to be investigated. We further hope that the similar shapes of some of our scaffolds to those of FDA-approved drugs will increase the likelihood of discovering biologically relevant molecules by using this method.
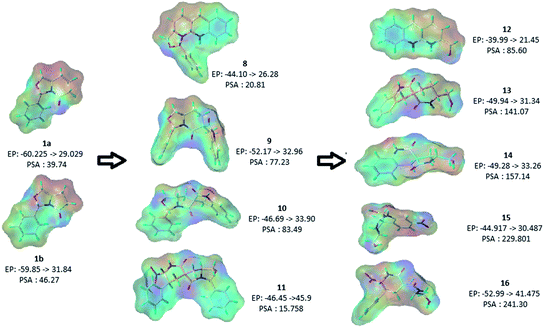
The polar surface area (PSA) of a molecule is the surface sum of all polar atoms, viz. oxygen and nitrogen, and hydrogens attached to them. In pharmaceutical chemistry it is a commonly used metric for evaluating drug's cell permeability and an important contributor to ligand/receptor binding at active sites. In general molecules with a PSA > 140 Å is poor in permeating cell membranes. For molecules with PSA < 60 Å are more likely to penetrate the blood–brain barrier and are preferred to act on receptors in the central nervous system. In order to assess the diversity of these scaffolds w.r.t their polar surface area we calculated the electrostatic profiles of the surfaces of our molecules by projecting the Gasteiger–Marsili charge-distribution onto the Connolly surface that was generated by using the MOLCAD tool in SYBYL and plotting the PSA distributions.15 A wide distribution of PSAs from 15 Å to 240 Å in addition, to diverse surface electrostatic potential maps indicate diverse shapes and electron-densities that allow these scaffolds to be potential biological modulators across a wide spectrum of therapeutic targets (Fig. 2).
Finally in a bid to evaluate the diversity quotient of our scaffold library (green squares) we compared them in scattered plots against randomly selected compounds from FDA approved drugs (red circles), macrocyclic natural products (solid blue triangles) natural product database (blue triangles) and from commercial databases such as ChemDiv (green cross) and ChemBridge (blue square). The incorporation of these additional compound sets in the analysis enables us to compare the chemical space covered by the collections and also provides some perspective to the positions of the library compounds in chemical space with respect to known biologically active regions. 2D molecular descriptors (via MOE) viz. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P, PSA, HBA (hydrogen bond acceptor), HBD (hydrogen bond donor) and etc. of all these compounds were calculated, followed by principal component analysis (PCA) of the values were obtained.16 Fig. 3 exhibits a scatter plot of the compound collections. From the plots it is quite obvious that our library compounds cover a relatively large area of chemical space, indicating the successful incorporation of a high degree of molecular diversity into the synthesis.
P, PSA, HBA (hydrogen bond acceptor), HBD (hydrogen bond donor) and etc. of all these compounds were calculated, followed by principal component analysis (PCA) of the values were obtained.16 Fig. 3 exhibits a scatter plot of the compound collections. From the plots it is quite obvious that our library compounds cover a relatively large area of chemical space, indicating the successful incorporation of a high degree of molecular diversity into the synthesis.
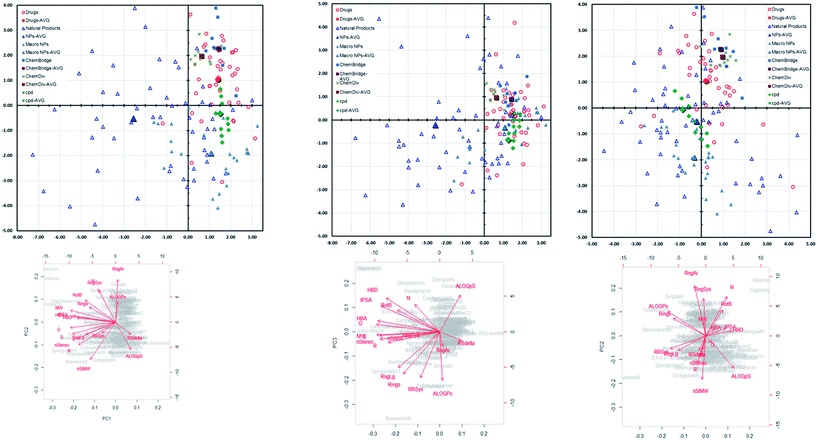 | ||
| Fig. 3 Principal Component Analysis (PCA) to assess the diversity of our library against various chemical database. | ||
Conclusions
Herein we have reported B/C/P based facile DOS synthesis of novel and privileged spiro- and fused scaffolds from pyrroldine and piperidine based chiral auxiliaries. The diversity of scaffold library when assessed via PMI and PCA against FDA approved drugs demonstrated a wide coverage of chemical space. A post pairing phase generated scaffolds with polar handle that facilitates further proliferation of these scaffolds into novel drug like compounds. The apogee of our methodology is a three-step synthesis of ten distinct natural-product-inspired scaffolds. This methodology has excellent steps per scaffold efficiency of 1.7 whilst generating chemically complex molecules (Scheme 5).Experimental section
General information
All reactions were carried out under an atmosphere of nitrogen in flame-dried glassware with a magnetic stirrer. Reactive liquids were transferred by syringe or cannula and were charged to the reaction flask through rubber septa. Common chemicals were obtained from Sigma-Aldrich Chemical Company and used without purification. Compounds 1a, 1b, 2a, 2b, 3 and 6 were prepared by a standard literature protocol.16 Lithium hexamethyldisilylamide (LDA) was obtained from Spectrochem Ltd. and used without purification. Tetrahydrofuran (THF) was freshly distilled prior to use from sodium and benzophenone ketyl under nitrogen and methylene chloride was freshly distilled from calcium hydride under nitrogen. Analytical thin layer chromatography was performed with Merck silica gel plates (0.25 mm thickness) with PF254 indicator. Compounds were visualized under UV lamp or by iodine treatment. Column chromatography was carried out on 60–120 mesh silica gel with technical grade solvents, which were distilled prior to use. 1H NMR spectra were recorded on Bruker 300 Ultrashield™ at 300 MHz. 13C NMR spectra were obtained on the same instrument at 75.5 MHz in CDCl3 solution with tetramethylsilane (1H NMR spectra) and [D6]DMSO or CDCl3 (13C NMR spectra) as an internal reference, unless otherwise stated. Chemical shifts (δ) are reported in ppm. All 13C NMR spectra were measured with complete proton decoupling. Data are reported as follows: s = singlet, d = doublet, t = triplet, m = multiplet.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30). 1H NMR (400 MHz, CDCl3): δ 1.95–2.00 (m, 1H), 2.61–2.81 (m, 2H), 3.8 (s, 3H), 3.91–3.92 (m, 1H), 4.11–4.18 (m, 1H), 4.21–4.31 (t, J = 2.6 Hz, 1H), 4.95–5.00 (m, 1H), 5.29–5.40 (m, 1H), 6.21 (s, 1H), 7.23–7.40 (m, 9H); 13C NMR (100 MHz, CDCl3): δ 32.7, 46.9, 53.4, 55.8, 63.5, 71.3, 86.9, 87.1, 125.9, 126.0, 128.5, 128.7, 128.8, 128.86, 128.9, 129.22, 129.25, 135.4, 137.5, 170.4, 171.7; HRMS (ES+) exact mass calculated for [M + H]+ (C22H23N2O6) requires (m/z) 411.1478, found (m/z) 411.1476.
30). 1H NMR (400 MHz, CDCl3): δ 1.95–2.00 (m, 1H), 2.61–2.81 (m, 2H), 3.8 (s, 3H), 3.91–3.92 (m, 1H), 4.11–4.18 (m, 1H), 4.21–4.31 (t, J = 2.6 Hz, 1H), 4.95–5.00 (m, 1H), 5.29–5.40 (m, 1H), 6.21 (s, 1H), 7.23–7.40 (m, 9H); 13C NMR (100 MHz, CDCl3): δ 32.7, 46.9, 53.4, 55.8, 63.5, 71.3, 86.9, 87.1, 125.9, 126.0, 128.5, 128.7, 128.8, 128.86, 128.9, 129.22, 129.25, 135.4, 137.5, 170.4, 171.7; HRMS (ES+) exact mass calculated for [M + H]+ (C22H23N2O6) requires (m/z) 411.1478, found (m/z) 411.1476.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30). 1H NMR (300 MHz, CDCl3): δ 1.62–2.15 (m, 5H), 2.72–2.85 (m, 3H), 3.42–3.52 (m, 1H), 3.78–3.81 (m, 1H), 3.95 (s, 1H), 7.25–7.30 (m, 5H), 7.42–7.48 (m, 1H), 7.51–7.59 (m, 1H), 7.82 (s, 1H), 8.12–8.15 (d, J = 4 Hz, 1H), 8.88 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 151.7, 134.3, 134.2, 129.2, 128.8, 128.6, 127.3, 127.4, 127.0, 62.7, 57.5, 50.9, 33.0, 29.7, 29.6; LCMS (ES+) exact mass calculated for [M + H]+ (C20H22N2O) requires (m/z) 307.1732 found (m/z) 306.94.
30). 1H NMR (300 MHz, CDCl3): δ 1.62–2.15 (m, 5H), 2.72–2.85 (m, 3H), 3.42–3.52 (m, 1H), 3.78–3.81 (m, 1H), 3.95 (s, 1H), 7.25–7.30 (m, 5H), 7.42–7.48 (m, 1H), 7.51–7.59 (m, 1H), 7.82 (s, 1H), 8.12–8.15 (d, J = 4 Hz, 1H), 8.88 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 151.7, 134.3, 134.2, 129.2, 128.8, 128.6, 127.3, 127.4, 127.0, 62.7, 57.5, 50.9, 33.0, 29.7, 29.6; LCMS (ES+) exact mass calculated for [M + H]+ (C20H22N2O) requires (m/z) 307.1732 found (m/z) 306.94.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25). 1H NMR (300 MHz, CDCl3): δ 1.51–1.72 (m, 2H), 1.95–2.15 (m, 4H), 2.81–2.88 (d, J = 24 Hz, 1H), 3.58–3.68 (t, J = 12 Hz, 1H), 3.78–3.82 (d, J = 18 Hz, 1H), 3.95–4.12 (m, 1H), 4.32–4.39 (t, J1 = 12 Hz, J2 = 8 Hz, 1H), 6.45 (s, 1H), 6.78–6.88 (d, J = 8 Hz, 1H), 6.98–7.02 (m, 1H), 7.16–7.19 (m, 2H), 7.28–7.42 (m, 3H), 7.51–7.58 (m, 2H), 8.15 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 171.15, 167.30, 137.99, 135.96, 128.88, 128.68, 128.50, 127.69, 126.73, 126.40, 123.46, 121.54, 114.95, 89.25, 72.64, 56.30, 50.10, 34.72, 28.01, 22.50; LCMS (ES+) exact mass calculated for [M + H]+ (C21H20N2O3) requires (m/z) 349.1474, found (m/z) 349.0.
25). 1H NMR (300 MHz, CDCl3): δ 1.51–1.72 (m, 2H), 1.95–2.15 (m, 4H), 2.81–2.88 (d, J = 24 Hz, 1H), 3.58–3.68 (t, J = 12 Hz, 1H), 3.78–3.82 (d, J = 18 Hz, 1H), 3.95–4.12 (m, 1H), 4.32–4.39 (t, J1 = 12 Hz, J2 = 8 Hz, 1H), 6.45 (s, 1H), 6.78–6.88 (d, J = 8 Hz, 1H), 6.98–7.02 (m, 1H), 7.16–7.19 (m, 2H), 7.28–7.42 (m, 3H), 7.51–7.58 (m, 2H), 8.15 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 171.15, 167.30, 137.99, 135.96, 128.88, 128.68, 128.50, 127.69, 126.73, 126.40, 123.46, 121.54, 114.95, 89.25, 72.64, 56.30, 50.10, 34.72, 28.01, 22.50; LCMS (ES+) exact mass calculated for [M + H]+ (C21H20N2O3) requires (m/z) 349.1474, found (m/z) 349.0.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25). 1H NMR (300 MHz, DMSO-D6): δ 2.15–2.21 (m, 1H), 2.59–2.69 (m, 2H), 3.16–3.20 (m, 1H), 3.51–3.61 (t, J1 = 6 Hz, J2 = 8 Hz, 1H), 4.18–4.38 (m, 2H), 6.12 (s, 1H), 6.82–6.95 (m, 2H), 7.05–7.19 (m, 2H), 7.32–7.42 (m, 5H), 10.44 (s, 1H); 13C NMR (100 MHz, DMSO-D6) 177.5, 140.2, 138.8, 130.1, 129.7, 129.3, 128.9, 127.4, 124.1, 122.5, 116.0, 88.6, 72.7, 57.6, 56.5, 49.8, 49.7; LCMS (ES+) exact mass calculated for [M + H]+ (C20H18N2O3) requires (m/z) 335.1317, found (m/z) 335.01.
25). 1H NMR (300 MHz, DMSO-D6): δ 2.15–2.21 (m, 1H), 2.59–2.69 (m, 2H), 3.16–3.20 (m, 1H), 3.51–3.61 (t, J1 = 6 Hz, J2 = 8 Hz, 1H), 4.18–4.38 (m, 2H), 6.12 (s, 1H), 6.82–6.95 (m, 2H), 7.05–7.19 (m, 2H), 7.32–7.42 (m, 5H), 10.44 (s, 1H); 13C NMR (100 MHz, DMSO-D6) 177.5, 140.2, 138.8, 130.1, 129.7, 129.3, 128.9, 127.4, 124.1, 122.5, 116.0, 88.6, 72.7, 57.6, 56.5, 49.8, 49.7; LCMS (ES+) exact mass calculated for [M + H]+ (C20H18N2O3) requires (m/z) 335.1317, found (m/z) 335.01.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30). 1H NMR (300 MHz, CDCl3): δ 1.72–1.98 (m, 4H), 2.72–2.85 (m, 3H), 3.42–3.52 (m, 1H), 3.81 (s, 2H), 7.51–7.59 (m, 1H), 7.61–7.68 (m, 1H), 7.72–7.78 (m, 1H), 7.81–7.84 (m, 1H), 8.22–8.32 (m, 1H), 9.41 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 128.8, 128.5, 127.4, 127.3, 126.4, 124.6, 124.5, 58.2, 55.9, 33.7, 33.1, 27.4, 25.8, 25.2, 23.2; LCMS (ES+) exact mass calculated for [M + H]+ (C13H18N2O) requires (m/z) 219.1419, found (m/z) 219.04.
30). 1H NMR (300 MHz, CDCl3): δ 1.72–1.98 (m, 4H), 2.72–2.85 (m, 3H), 3.42–3.52 (m, 1H), 3.81 (s, 2H), 7.51–7.59 (m, 1H), 7.61–7.68 (m, 1H), 7.72–7.78 (m, 1H), 7.81–7.84 (m, 1H), 8.22–8.32 (m, 1H), 9.41 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 128.8, 128.5, 127.4, 127.3, 126.4, 124.6, 124.5, 58.2, 55.9, 33.7, 33.1, 27.4, 25.8, 25.2, 23.2; LCMS (ES+) exact mass calculated for [M + H]+ (C13H18N2O) requires (m/z) 219.1419, found (m/z) 219.04.Acknowledgements
We acknowledge GVK Bioscience for financial support.References
- (a) G. B. Bauer and L. F. Povirk, Nucleic Acids Res., 1997, 25, 1211–1218 CrossRef CAS PubMed; (b) L. H. Hurley, Nat. Rev. Cancer, 2002, 2, 188–200 CrossRef CAS PubMed; (c) J. Reedijk, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 3611–3616 CrossRef CAS PubMed; (d) B. Clement and F. N. Jung, Drug Metab. Dispos., 1994, 22, 486–497 CAS; (e) W. A. Remers, The Chemistry of Antitumor Antibiotics, Wiley, New York, 1979, p. 1 Search PubMed; (f) L. D. Williams, M. Egli, G. Qi, P. Bash, G. A. van der Marel, J. H. van Boom, A. Rich and C. A. Frederick, Proc. Natl. Acad. Sci. U. S. A., 1990, 87, 2225–2229 CrossRef CAS; (g) R. Sinha, M. Hossain and G. S. Kumar, DNA Cell Biol., 2009, 28(4), 209–219 CrossRef CAS PubMed; (h) E. H. Bayne and R. C. Allshire, Trends Genet., 2005, 21, 370 CrossRef CAS PubMed; (i) M. C. Jovanovic and M. O. Hengartner, Oncogene, 2006, 25, 6176 CrossRef CAS PubMed; (j) G. L. Sen and H. M. Blau, FASEB J., 2006, 20, 1293 CrossRef CAS PubMed; (k) L. Manche, S. R. Green, C. Schmedt and M. B. Mathews, Mol. Cell. Biol., 1992, 12, 5238 CAS; (l) C. A. Sledz, M. Holko, M. J. de Veer, R. H. Silverman and B. R. Williams, Nat. Cell Biol., 2003, 5, 834 CrossRef CAS PubMed; (m) M. J. Fedor and J. R. Williamson, Nat. Rev. Mol. Cell Biol., 2005, 6, 399 CrossRef CAS PubMed; (n) J. Drews, Nat. Biotechnol., 1996, 14, 1516 CrossRef CAS PubMed; (o) J. Drews and S. Ryser, Nat. Biotechnol., 1997, 15, 1318 CrossRef CAS PubMed; (p) A. L. Hopkins and C. R. Groom, Nat. Rev. Drug Discovery, 2002, 1, 727 CrossRef CAS PubMed; (q) D. S. Wishart, C. Knox, A. C. Guo, S. Shrivastava, M. Hassanali, P. Stothard, P. Z. Chang and J. Woolsey, Nucleic Acids Res., 2006, 34, D668 CrossRef CAS PubMed.
- (a) T. E. Nielsen and S. L. Schreiber, Angew. Chem., Int. Ed., 2008, 47, 48–56 CrossRef CAS PubMed; (b) B. E. Evans, K. E. Rittle, M. G. Bock, R. M. DiPardo, R. M. Freidinger, W. L. Whitter, G. F. Lundell, D. F. Veber, P. S. Anderson, R. S. L. Chang, V. J. Lotti, D. J. Cerino, T. B. Chen, P. J. Kling, K. A. Kunkel, J. P. Springer and J. Hirshfield, J. Med. Chem., 1988, 31, 2235–2246 CrossRef CAS; (c) S. L. Schreiber, Science, 2000, 287, 1964–1969 CrossRef CAS.
- (a) G. L. Thomas, R. J. Spandl, F. G. Glansdrop, M. Welch, A. Bender, J. Cockfield, J. A. Lindsay, C. Bryant, D. F. Brown, O. Loiseleur, H. Rudyk, M. Ladlow and D. R. Spring, Angew. Chem., 2008, 120, 2850–2854 (Angew. Chem., Int. Ed., 2008, 47, 2808–2812) CrossRef; (b) H. An, S.-J. Eum, M. Koh, S. K. Lee and S. B. Park, J. Org. Chem., 2008, 73, 1752–1761 CrossRef CAS PubMed; (c) H. E. Pelish, N. J. Westwood, Y. Feng, T. Kirchhausen and M. Shair, J. Am. Chem. Soc., 2001, 123, 6740–6741 CrossRef CAS; (d) D. B. Ramachary, C. Venkaiah, Y. V. Reddy and M. Kishor, Org. Biomol. Chem., 2009, 7, 2053–2062 RSC.
- (a) T. Uchida, M. Rodriquez and S. L. Schreiber, Org. Lett., 2009, 11(7), 1559–1562 CrossRef CAS PubMed; (b) M. E. Fitzgerald, C. A. Mulrooney, J. R. Duvall, J. Wei, B.-C. Suh, L. B. Akella, A. Vrcic, L. A. Marcaurelle, E. Ascic, S. T. Le Quement, M. Ishoey, M. Daugaard and T. E. Nielsen, ACS Comb. Sci., 2012, 14(4), 253–257 CrossRef PubMed.
- (a) C. E. Mills née Davis, T. D. Heightman, S. A. Hermitage, M. G. Moloney and G. A. Woods, Tetrahedron Lett., 1998, 39(9), 1025–1028 CrossRef; (b) S. A. Hermitage and M. G. Moloney, Tetrahedron: Asymmetry, 1994, 5(8), 1463–1464 CrossRef CAS; (c) J. H. Bailey, A. T. J. Byfield, P. J. Davis, A. C. Foster, K. Prout, M. Leech, M. G. Moloney and M. Mueller, Perkin 1, 2000, 12, 1977–1982 RSC.
- S. Sen, V. R. Potti, R. Surakanti, Y. L. N. Murthy and R. Pallepogu, Org. Biomol. Chem., 2011, 9, 358–360 CAS.
- V. S. B. Damerla, C. Tulluri, R. Gundla, L. Naviri, U. Adepally, P. S. Iyer, Y. L. N. Murthy, N. Prabhakar and S. Sen, Chem. – Asian J., 2012, 7, 2351–2360 CrossRef CAS PubMed.
- (a) J. E. Baldwin, M. G. Moloney and S. B. Shim, Tetrahedron Lett., 1991, 32(10), 1379–1380 CrossRef CAS; (b) J. H. Bailey, M. J. Beard, T. D. Cherry, M. G. Moloney, S. B. Shim, K. A. Statham, M. J. Bamford and B. R. Lamont, Tetrahedron, 1996, 52(10), 3719–3740 CrossRef; (c) A. R. Cowley, T. J. Hill, M. G. Moloney, R. D. Stevenson, A. L. Thomson and P. Kocis, Org. Biomol. Chem., 2011, 9(20), 7042–7056 RSC; (d) M. Amat, J. Hidlago and J. Bosch, Tetrahedron: Asymmetry, 1996, 7(6), 1591–1594 CrossRef CAS; (e) M. Amat, N. Llor, J. Hidalgo, J. Bosch, E. Molins and C. Miravitlles, Tetrahedron: Asymmetry, 1996, 7(9), 2501–2504 CrossRef CAS.
- (a) H. Jhoti, G. Williams, D. C. Rees and C. W. Murray, Nat. Rev. Drug Discovery, 2013, 12, 644–679 CrossRef CAS PubMed; (b) M. Baker, Nat. Rev. Drug Discovery, 2013, 12, 5–7 CrossRef CAS PubMed; (c) M. Congreve, R. Carr, C. Murray and H. Jhoti, Drug Discovery Today, 2003, 8, 867–877 CrossRef.
- (a) J. E. DeLorbe, D. Horne, R. Jove, S. M. Mennen, S. Nam, F.-L. Zhang and L. E. Overman, J. Am. Chem. Soc., 2013, 135(10), 4117–4128 CrossRef CAS PubMed; (b) L. E. Overman and Y. Shin, Org. Lett., 2007, 9(2), 339–341 CrossRef CAS PubMed; (c) H. Song, J. Yang, W. Chen and Y. Qin, Org. Lett., 2006, 8(26), 6011–6014 CrossRef CAS PubMed; (d) A. Coste, J. Kim, T. C. Adams and M. Movassaghi, Chem. Sci., 2013, 4, 3191–3197 RSC; (e) Q. Wei, Y.-Y. Wang, Y.-L. Du and L.-Z. Gong, Beilstein J. Org. Chem., 2013, 9, 1559–1564 CrossRef PubMed; (f) M. S. Morales-Rios, N. F. Santos-Sanchez and P. Joseph-Nathan, J. Nat. Prod., 2002, 65(2), 136–141 CrossRef CAS PubMed; (g) P. L. Julian and J. Pikl, J. Am. Chem. Soc., 1934, 56(8), 1797–1801 CrossRef CAS; (h) P. L. Julian and J. Pikl, J. Am. Chem. Soc., 1935, 57(3), 539–544 CrossRef CAS.
- (a) P.-Q. Huang, Z.-Q. Guo and Y.-P. Ruan, Org. Lett., 2006, 8(7), 1435–1438 CrossRef CAS PubMed; (b) S. M.-C. Pelletier, P. C. Ray and D. J. Dixon, Org. Lett., 2009, 11(20), 4512–4515 CrossRef CAS PubMed; (c) F. Xu, E. Corley, J. A. Murry and D. M. Tschaen, Org. Lett., 2007, 9(14), 2669–2672 CrossRef CAS PubMed; (d) P. S. Hynes, P. A. Stupple and D. J. Dixon, Org. Lett., 2008, 10(7), 1389–1391 CrossRef CAS PubMed.
- (a) R. M. Sweet and L. F. Dahl, J. Am. Chem. Soc., 1970, 92(18), 5489–5507 CrossRef CAS; (b) L. Pauling, Chem. Eng. News, 1946, 24(10), 1375–1377 CrossRef CAS PubMed; (c) F. Diederich, Chimia, 2001, 55, 821–827 CAS.
- W. H. B. Sauer and M. K. Schwartz, J. Chem. Inf. Model., 2003, 43, 987–1003 CrossRef CAS PubMed.
- Proprietary Database of GVK Bioscience, 28A, IDA Nacharam, Hyderabad, Andhra Pradesh, India.
- H. Pajouhesh and G. R. Lenz, NeuroRx, 2005, 2, 541–553 CrossRef PubMed.
- Stable 3D structures of all compounds were used to calculate their 2D-med-chem distributors, such as molecular weight, number of hydrogen-bond donors (HBD), number of hydrogen bond acceptors (HBA), A
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P, log
P, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D, polar surface area (PSA) and etc., these values were calculated by using Discovery Studio 3.1 (Accelrys Inc.).
D, polar surface area (PSA) and etc., these values were calculated by using Discovery Studio 3.1 (Accelrys Inc.).
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra47714b |
| This journal is © The Royal Society of Chemistry 2014 |