One-step solution-phase synthesis of Co3O4/RGO/acetylene black as a high-performance catalyst for oxygen reduction reaction
Qunfeng Wangab,
Huimin Yuana,
Haibo Fenga,
Junhua Lia,
Chao Zhaoa,
Jinlong Liua,
Dong Qian*ab,
Jianbo Jianga and
Youcai Liu*a
aCollege of Chemistry and Chemical Engineering, Central South University, Changsha 410083, P. R. China. E-mail: qiandong6@vip.sina.com; liuyoucai@126.com; Fax: +86-731-88879616; Tel: +86-731-88879616
bState Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, P. R. China
First published on 26th March 2014
Abstract
On the way to become promising oxygen reduction reaction (ORR) catalysts, the hybrids composed of reduced graphene oxide (RGO) and transition metal oxides are suffering from stacking of RGO sheets. In this work, a Co3O4/RGO/acetylene black (AB) hybrid was successfully synthesized via a facile one-step solution-phase route with sandwiching of AB particles between the RGO sheets during the synthesis of Co3O4/RGO, which can effectively tackle the stacking of RGO sheets. Compared with Co3O4/RGO, Co3O4/RGO/AB-P (mixing AB with the pre-prepared Co3O4/RGO with stirring), Co3O4/RGO/AB-M (mixing AB with Co3O4/RGO during the fabrication of the Co3O4/RGO catalytic layer for ORR) and commercial 10 wt% Pt/C, the Co3O4/RGO/AB hybrid exhibits increases of 50.6%, 32.5%, 37.9% and 8.9% in the ORR current density, respectively. This indicates that the introduction strategy of AB to Co3O4/RGO plays a vital role in the enhancement of ORR catalytic activity. Moreover, the Co3O4/RGO/AB hybrid shows a subtle ascending trend in the ORR current density during continuous operation for 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s, while Pt/C exhibits a 9.0% decrease. The exceptional ORR catalytic performance of Co3O4/RGO/AB can also be ascribed to the large specific surface area, well-anchored Co3O4 nanoparticles on the RGO sheets, and low ohmic and kinetic impedances for ORR. We hope this work will be conducive for the extensive commercial applications of fuel cells.
000 s, while Pt/C exhibits a 9.0% decrease. The exceptional ORR catalytic performance of Co3O4/RGO/AB can also be ascribed to the large specific surface area, well-anchored Co3O4 nanoparticles on the RGO sheets, and low ohmic and kinetic impedances for ORR. We hope this work will be conducive for the extensive commercial applications of fuel cells.
1. Introduction
The extremely expensive cost of fuel cells, mainly caused by the exclusive use of Pt and Pt-based catalysts for oxygen reduction reaction (ORR), has become the biggest barrier for the extensive commercial application of fuel cells.1–3 Therefore, during the last decade, tremendous efforts have been directed to develop effective, inexpensive and environmentally friendly ORR catalysts that substitute the expensive Pt or Pt-based catalysts in fuel cells.4,5 Alternative carbon materials,6,7 transition metal oxides (MxOy),8,9 perovskite oxides10,11 and pyrolized metal porphyrins12,13 have greatly attracted the attention of researchers due to their low costs, high stabilities and relatively good catalytic activities. Graphene, with a unique two-dimensional sp2-hybridized single layer carbon structure, has been regarded as a very appealing energy storage material because of its excellent properties, such as large theoretical specific surface area, extraordinarily high conductivity, outstanding mechanical flexibility and superb chemical stability.14−18 Among these studies, it has been reported that some hybrids constituted by graphene and MxOy exhibited excellent ORR catalytic performances comparable to commercial Pt/C catalyst.19−21Although Co3O4 or reduced graphene oxide (RGO) alone has little catalytic activity, Liang et al. pioneered the use of a Co3O4/RGO hybrid as ORR catalyst, which exhibited an unexpected and surprisingly high ORR activity.22 However, it is well known that the RGO sheets tend to form irreversible agglomerates or even restack to form graphite through van der Waals interactions in the production of RGO via chemical conversion or thermal expansion/reduction.23,24 Moreover, some reports pointed out that agglomerating or stacking also occurs when RGO sheets are dried (even when they are loaded with nanoparticles), which will inevitably block a great number of catalytic sites on nanoparticles and set a substantial barrier for the diffusion of reactant molecules, leading to retardation of the catalytic reaction.25,26 Therefore, alleviating the degree of RGO stacking is one of the highly desirable strategies to maximize the use of RGO and its hybrids in catalysis.
Recently, considerable scientific efforts have been made to mitigate the degree of RGO stacking. Hu et al. proposed a powerful route to fabricate N-doped RGO via functionalization by self-assembled molecules, ultra-rapid thermal expansion–exfoliation, and covalent transformation. In this method, the RGO stacking could be effectively attenuated through the ultrafast thermal expansion to produce unique mesoporous structures and thus a high specific surface area could be obtained.27 Hu et al. also designed an effective strategy to inhibit the aggregation of RGO sheets by introducing one-dimensional carbon nanotubes to form 3-D hierarchical structure.28 Chang et al. demonstrated an approach to alleviate RGO stacking by precipitating hydroxides onto GO sheets followed by microwave-assisted hydrothermal/solvothermal annealing.29 Si et al. tried to reduce the RGO stacking by introducing sulfonic acid groups in RGO.30 Moreover, they synthesized Pt/RGO with 3–4 nm Pt crystallites on the RGO surfaces to minimize RGO stacking.23
Li et al. tried mixing carbon black (CB) particles with Pt-loaded RGO.25 The results show that with the insertion of CB particles between RGO sheets, stacking of RGO can be effectively prevented and ORR electrocatalytic performances of the Pt/RGO/CB hybrid can be greatly enhanced. This work provides a hint to us for further improvement of the ORR electrocatalytic performances of Co3O4/RGO. However, we noted that Li et al. mixed the CB particles with Pt/RGO via a time-consuming physical method, in which the CB particles were loaded on the pre-synthesized Pt/RGO with stirring overnight. As mentioned above, stacking of RGO sheets is involved in the whole preparation procedure, and some reports have demonstrated that metal ions or metal salts can also induce stacking of RGO and graphene oxide (GO) sheets.31 Once stacking of RGO is complete, we can imagine that it is difficult for the CB particles to completely insert between the RGO sheets merely by simple physical mixing; as a result, the advantages of RGO cannot function to the utmost. Thus, we think that there exists some scope to alleviate RGO stacking by improving the way CB particles are introduced into the RGO-based hybrids.
Herein, we successfully synthesized the Co3O4/RGO/acetylene black (AB) hybrid through introduction of AB to the hybrid concurrent with reduction of GO to RGO, formation of Co3O4, and deposition of Co3O4 on RGO via a one-step solution-phase route. Compared with Co3O4/RGO, Co3O4/RGO/AB-P (mixing AB with the pre-prepared Co3O4/RGO with stirring), Co3O4/RGO/AB-M (mixing AB with Co3O4/RGO during the fabrication of the Co3O4/RGO catalytic layer for ORR) and commercial 10 wt% Pt/C, the as-prepared Co3O4/RGO/AB hybrid exhibits a significantly enhanced ORR catalytic activity. To the best of our knowledge, no studies have yet been reported on the Co3O4/RGO/AB hybrid with the insertion of AB particles between RGO sheets and the way AB is introduced during the preparation of Co3O4/RGO.
2. Experimental
2.1 Synthesis of GO
GO was synthesized based on modified Hummers' methods32,33 with some changes. In a typical experiment, 1 g of graphite flakes were grounded with 20 g of NaCl for 15 min, and then NaCl was removed by washing with deionized water under vacuum filtration. The obtained wet graphite was dried at 70 °C for 30 min, and then transferred to a 250 mL beaker containing 23 mL of concentrated H2SO4, followed by magnetic stirring for 24 h. Afterwards, the suspension was heated to 35 °C, and 0.5 g of NaNO3 was added with magnetic stirring. About 5 min later, 1.5 g of KMnO4 was added slowly to the suspension so as to control the temperature of the mixture below 40 °C, and the mixture was kept at this temperature for 60 min with stirring without interruption. Then, 6 mL of deionized water was added slowly into the mixture followed by another 40 mL after 5 min. After that, the mixture was heated to 60 °C and kept for 15 min. Then, 140 mL of deionized water and 10 mL of 30% H2O2 were added to the mixture in sequence, and the mixture was kept stirring for 5 min. The precipitates were collected by centrifugation at 5000 rpm and washed with 5% HCl aqueous solution twice and deionized water three times. The as-received GO was dispersed in 1500 mL of anhydrous ethanol and sonicated for 60 min to obtain a GO suspension. In the end, the suspension was centrifuged at 5000 rpm for 15 min to remove the unoxidized graphite, and a brown uniform supernatant with a concentration of 0.5 mg mL−1 of GO was obtained.2.2 Synthesis of the Co3O4/RGO hybrid
The Co3O4/RGO hybrid was prepared according to Liang et al.'s method with some modifications.22 Typically, 14.4 mL of 0.2 M Co(Ac)2 aqueous solution and 4.6 mL of deionized water were added to 190 mL of the above GO anhydrous ethanol suspension in sequence, and then the mixture was kept at 80 °C for 10 h with stirring. Afterwards, the obtained mixture was divided into three parts and transferred to three 100 mL Teflon-lined stainless steel autoclaves, sealed and heated at 150 °C for 3 h for the purposes of reduction of GO to RGO, formation of Co3O4, and deposition of Co3O4 on RGO. Finally, the obtained composite was collected by centrifugation at 3000 rpm, washed with ethanol three times and deionized water twice, and lyophilizated to get the Co3O4/RGO hybrid.2.3 Synthesis of the Co3O4/RGO/AB hybrid
The synthesis of the Co3O4/RGO/AB hybrid was similar to that of the Co3O4/RGO hybrid except that the addition of 50 mL of 1.4 mg mL−1 AB anhydrous ethanol suspension was concurrent with the addition of 14.4 mL of 0.2 M Co(Ac)2 aqueous solution into 190 mL of GO anhydrous ethanol suspension for the preparation of Co3O4/RGO/AB.2.4 Synthesis of the Co3O4/RGO/AB-P and Co3O4/RGO/AB-M hybrids
For comparison, the Co3O4/RGO/AB-P hybrid was synthesized by stirring the mixture of the pre-synthesized Co3O4/RGO with AB in isopropanol overnight by referring to the preparation of Pt/RGO/CB proposed by Li et al.25 Typically, 70 mg of AB was initially washed with isopropanol three times, and then dispersed in isopropanol with a concentration of 4 mg mL−1, followed by sonication for 30 min. Next, 250 mg of the pre-obtained Co3O4/RGO catalyst was dispersed in isopropanol with a concentration of 0.1 mg mL−1, and then it was mixed with the as-obtained AB suspension. The as-received mixture was sonicated for 30 min and was magnetically stirred overnight. In the end, ∼320 mg of Co3O4/RGO/AB-P hybrid was collected after centrifugation at 8000 rpm and lyophilization. Moreover, in order to further understand the role of AB in Co3O4/RGO/AB, Co3O4/RGO/AB-M hybrid was prepared through the addition of the same amount AB in the blending process during the manufacture of the catalytic layer loaded with Co3O4/RGO in the assembly of the gas-diffusion electrode (GDE) as described in 2.6. In the Co3O4/RGO hybrid, Co3O4 was ∼70% by mass and RGO was ∼30%. In the Co3O4/RGO/AB and Co3O4/RGO/AB-P hybrids, Co3O4 was ∼55% by mass, RGO was ∼23% and AB was ∼22%.2.5 Characterizations
The phase identifications of samples were performed by powder X-ray diffraction (XRD) on a Rigaku D/max-2500 X-ray diffractometer with Cu Kα radiation (λ = 1.54056 Å). The microstructures and morphologies of samples were observed by a scanning electron microscope (SEM, JEOL JSM-5612LV) with an accelerating voltage of 10 kV and a transmission electron microscope (TEM, JEOL-2010) with an accelerating voltage of 200 kV. The HAADF (high-angle annular dark field) image and energy dispersive X-ray (EDX) mapping were performed in STEM mode on a Titan G2 60-300 transmission electron microscope. The Brunauer–Emmett–Teller (BET) surface areas of samples were measured by nitrogen adsorption–desorption measurements on a Monosorb Autosorb analyzer. The total mass ratios of RGO and AB in hybrids were measured through thermogravimetric analysis (TGA) carried out on a Netzsch-Gerätebau GmbH-STA 449 C Jupiter thermo-microbalance.2.6 Electrode preparations and electrochemical measurements
The GDEs were assembled by sandwiching a gas diffusion layer and a catalytic layer together with a nickel wire screen.34 To prepare the catalytic layer, 80 wt% of catalyst powder was blended with 20 wt% of PTFE suspension (10 wt% of PTFE in H2O) in anhydrous ethanol by ultrasonic agitation, and then dried at 80 °C to get a dough-like paste. After that, the paste was rolled into a ∼0.2 mm thick layer and the catalyst was loaded at ∼1.6 mg cm−2. The gas diffusion layer consisting of AB and PTFE with a weight ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 was made in the same way. Finally, the gas diffusion and catalytic layers were rolled together with a nickel wire screen. Followed by drying at 60 °C, a ∼0.4 mm thick GDE was obtained in the end. In addition, the same amount AB in Co3O4/RGO/AB was added in the blending process during the manufacture of the catalytic layer loaded with Co3O4/RGO (Co3O4/RGO/AB-M) for the purpose of further understanding the role of AB in Co3O4/RGO/AB. To highlight the excellent ORR catalytic performance of Co3O4/RGO/AB, a commercial Pt/C electrocatalyst (10 wt% Pt supported on carbon black) was employed as the baseline catalyst for comparison.
3 was made in the same way. Finally, the gas diffusion and catalytic layers were rolled together with a nickel wire screen. Followed by drying at 60 °C, a ∼0.4 mm thick GDE was obtained in the end. In addition, the same amount AB in Co3O4/RGO/AB was added in the blending process during the manufacture of the catalytic layer loaded with Co3O4/RGO (Co3O4/RGO/AB-M) for the purpose of further understanding the role of AB in Co3O4/RGO/AB. To highlight the excellent ORR catalytic performance of Co3O4/RGO/AB, a commercial Pt/C electrocatalyst (10 wt% Pt supported on carbon black) was employed as the baseline catalyst for comparison.
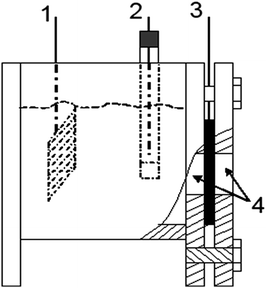
All electrochemical measurements of GDEs were conducted in a 6 M KOH aqueous solution in air at 25 °C. Fig. 1 illustrates the setup of a three-electrode configuration for the electrochemical measurements, in which the gas diffusion layer and the catalytic layer were exposed to air and electrolyte, respectively. The GDEs were cycled 30 times between 0 and −0.6 V to activate as many catalytic sites as possible. The oxygen reduction polarization curves of samples were recorded on a CHI660D electrochemical workstation at a scan rate of 2 mV s−1. The durability measurements of samples were also carried out on the CHI660D electrochemical workstation at −0.6 V. A PARSTAT 2273 advanced electrochemical system was employed to perform the electrochemical impedance spectroscopy (EIS) measurements of samples in the range from 1 Hz to 40 kHz with an amplitude of 10 mV. Finally, the Nyquist plots were adopted and further analyzed using ZSimpWin V3.10 to evaluate the properties of GDEs.
3. Results and discussion
Fig. 2 shows the XRD patterns of the as-synthesized Co3O4/RGO, Co3O4/RGO/AB and Co3O4/RGO/AB-P, and the pattern of PDF 61-2294 indicates the standard spectrum data for the face-centered cubic Co3O4 phase. Except for the wide weak diffraction peak at 2θ = 26.2° in Co3O4/RGO/AB, all other diffraction peaks of each sample coincide with JCPDS no. 61-2294 perfectly. The wide peak at 2θ = 26.2° in Co3O4/RGO/AB and Co3O4/RGO/AB-P is not found in Co3O4/RGO, implying that the peak belongs to AB. However, no diffraction peaks for carbon species can be observed in the XRD pattern of Co3O4/RGO, which might be ascribed to the relatively low diffraction intensity of graphene. Moreover, the Co3O4 particles grown on the RGO sheet can lower its graphitization degree.35,36The morphologies and microstructures of Co3O4/RGO, Co3O4/RGO/AB and Co3O4/RGO/AB-P were investigated by SEM and TEM. From the SEM images of Co3O4/RGO (Fig. 3a), Co3O4/RGO/AB (Fig. 3c) and Co3O4/RGO/AB-P (Fig. 3e), more divergent sheets and scattering particles can be clearly observed in Co3O4/RGO/AB. This indicates that the introduction of AB during the synthesis of Co3O4/RGO can effectively relieve the stacking of RGO sheets. This can be further confirmed by the BET surface areas of Co3O4/RGO, Co3O4/RGO/AB and Co3O4/RGO/AB-P of 84.22, 101.68 and 83.47 m2 g−1, respectively. This also suggests that mixing AB with the pre-prepared Co3O4/RGO with stirring has almost no effect on the specific surface area of Co3O4/RGO. From the TEM images of Co3O4/RGO (Fig. 3b), Co3O4/RGO/AB (Fig. 3d) and Co3O4/RGO/AB-P (Fig. 3f), we can easily find the two-dimensional structure of RGO sheets with wrinkles, which are anchored by a lot of nanoparticles. With the introduction of AB to Co3O4/RGO, the nanoparticles anchored on the RGO sheets become dense. As shown in the TEM image of Co3O4/RGO, Co3O4 nanoparticles present a roughly cubic morphology with sizes of 20–80 nm. However, a considerable number of Co3O4 nanoparticles grow outside the RGO sheets. Interestingly, Co3O4 and AB nanoparticles in Co3O4/RGO/AB are well anchored on the RGO sheets, which can be ascribed to the fact that sandwiching AB particles between the RGO sheets during the synthesis of Co3O4/RGO can provide a much larger surface area for accommodating nanoparticles. As for Co3O4/RGO/AB-P, however, we can observe the nanoparticles growing outside the RGO sheets. It is hard to distinguish the Co3O4 and AB particles from the above SEM or TEM images. To this end, the HAADF image and STEM-EDX mapping of Co3O4/RGO/AB were collected as shown in Fig. 3g and h, respectively, from which the Co3O4 and AB particles can be easily discriminated and the size of AB particles can be observed to be close to Co3O4 particles.
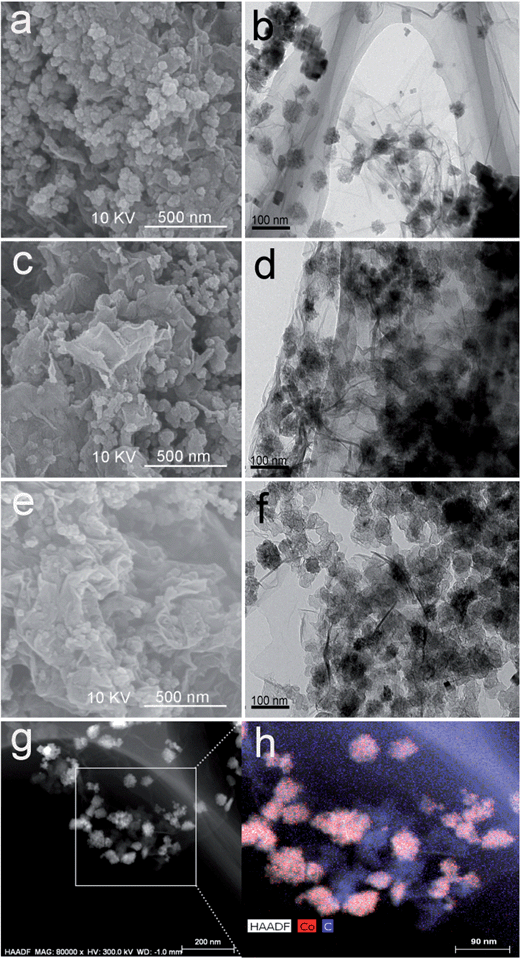 | ||
| Fig. 3 (a) SEM and (b) TEM images of Co3O4/RGO, (c) SEM and (d) TEM images of Co3O4/RGO/AB, (e) SEM and (f) TEM images of Co3O4/RGO/AB-P, and (g) HAADF image and (h) STEM-EDX mapping of Co3O4/RGO/AB. | ||
Fig. 4 depicts the cathode polarization curves and durability properties of the Co3O4/RGO, Co3O4/RGO/AB, Co3O4/RGO/AB-P, Co3O4/RGO/AB-M and Pt/C GDEs. As shown in Fig. 4a, the Co3O4/RGO, Co3O4/RGO/AB-M, Co3O4/RGO/AB-P, Pt/C and Co3O4/RGO/AB GDEs deliver ORR current densities of −412.3, −450.2, −468.5, −570.1 and −620.8 mA cm−2 at −0.6 V vs. Hg/HgO, respectively. Evidently, the Co3O4/RGO/AB GDE affords significantly high ORR current density among these GDEs, and displays increases of 50.6%, 37.9%, 32.5% and 8.9% compared with Co3O4/RGO, Co3O4/RGO/AB-M, Co3O4/RGO/AB-P and Pt/C GDEs, respectively. Further, considering that the Co3O4/RGO/AB-P GDE only exhibits a slight increase in the ORR current density compared with the Co3O4/RGO/AB-M GDE, it can be concluded that the way of AB introduction plays a vital role in the enhancement of ORR catalytic activity. Furthermore, the Co3O4/RGO/AB-P and Co3O4/RGO/AB-M GDEs show enhancements of only 13.6% and 9.2% in the ORR current density compared with the Co3O4/RGO GDE, respectively. It is well known that the durability of Pt-based ORR electrocatalysts is unsatisfactory.22 Surprisingly, as illustrated in Fig. 4b, all of these GDEs except Pt/C (exhibiting a 9.0% decrease) show subtle ascending trends in the ORR current density during continuous operation for 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s. This may be mainly due to the fact that the RGO sheets could work like fishing nets, and thus prevent the supported ingredients from dissolving into the electrolyte.37,38 This implies that the introduction of AB to Co3O4/RGO has a minor effect on the durability. As demonstrated by Liang et al., Co3O4 or RGO alone exhibits little ORR catalytic activity; however, their hybrid shows surprisingly high ORR catalytic activity, which can be attributed to the synergetic chemical coupling effects between Co3O4 and graphene.22 Therefore, the Co3O4 nanoparticles growing outside the RGO sheets as presented in the TEM images of Co3O4/RGO and Co3O4/RGO/AB-P contribute little to the ORR catalytic activity, leading to their relatively lower ORR current densities. On the other hand, as mentioned above, the way of AB introduction into Co3O4/RGO is essential to boost the ORR catalytic activity. It seems that inserting AB particles between the RGO sheets during the synthesis of Co3O4/RGO is much more efficacious to relieve stacking of RGO sheets than mixing AB with the pre-prepared Co3O4/RGO with stirring or during the fabrication of the Co3O4/RGO catalytic layer. As a result, the corresponding Co3O4/RGO/AB hybrid can provide far more channels for the diffusion of reactant molecules and offer a much larger RGO surface area for the growth of Co3O4 particles, which have been verified by the above SEM/TEM analyses and BET surface areas of corresponding hybrids.
000 s. This may be mainly due to the fact that the RGO sheets could work like fishing nets, and thus prevent the supported ingredients from dissolving into the electrolyte.37,38 This implies that the introduction of AB to Co3O4/RGO has a minor effect on the durability. As demonstrated by Liang et al., Co3O4 or RGO alone exhibits little ORR catalytic activity; however, their hybrid shows surprisingly high ORR catalytic activity, which can be attributed to the synergetic chemical coupling effects between Co3O4 and graphene.22 Therefore, the Co3O4 nanoparticles growing outside the RGO sheets as presented in the TEM images of Co3O4/RGO and Co3O4/RGO/AB-P contribute little to the ORR catalytic activity, leading to their relatively lower ORR current densities. On the other hand, as mentioned above, the way of AB introduction into Co3O4/RGO is essential to boost the ORR catalytic activity. It seems that inserting AB particles between the RGO sheets during the synthesis of Co3O4/RGO is much more efficacious to relieve stacking of RGO sheets than mixing AB with the pre-prepared Co3O4/RGO with stirring or during the fabrication of the Co3O4/RGO catalytic layer. As a result, the corresponding Co3O4/RGO/AB hybrid can provide far more channels for the diffusion of reactant molecules and offer a much larger RGO surface area for the growth of Co3O4 particles, which have been verified by the above SEM/TEM analyses and BET surface areas of corresponding hybrids.
 | ||
| Fig. 4 (a) Cathode polarization curves and (b) durability properties of the Co3O4/RGO, Co3O4/RGO/AB, Co3O4/RGO/AB-P, Co3O4/RGO/AB-M and Pt/C GDEs. | ||
In order to further confirm the advantages of Co3O4/RGO/AB over other hybrids and highlight the benefits of the fabrication strategy of Co3O4/RGO/AB, the EIS tests of the Co3O4/RGO, Co3O4/RGO/AB, Co3O4/RGO/AB-P and Co3O4/RGO/AB-M GDEs were carried out, and the corresponding Nyquist plots at the open-circuit potential (Eocp) and −0.2 V are presented in Fig. 5.
 | ||
| Fig. 5 Impedance spectra of the Co3O4/RGO, Co3O4/RGO/AB, Co3O4/RGO/AB-P and Co3O4/RGO/AB-M GDEs at (a) Eocp and (b) −0.2 V. | ||
The Nyquist plots can be divided into two regions, i.e., the high-frequency region and the low-frequency region. As shown in Fig. 5, semi-circles can be observed in the high-frequency region, of which sizes change little from Eocp to −0.2 V, indicating that this part of the impedance arises from the ohmic process in GDEs. The low-frequency region with the rapid decrease in size is the kinetic impedance of ORR.39 Since the current is only about −50 mA cm−2 at −0.2 V and the 45°-like straight lines appear in the low-frequency region of Nyquist plots, which reveal the existing of bounded diffusion,40 we did not place a diffusion element (W) in the equivalent circuit. Fig. 6 shows the equivalent circuit, where Rs is the electrolyte resistance between the reference electrode and the GDE, R1 is the ohmic resistance of GDE, R2 is the electrochemical charge-transfer resistance of ORR, and the constant-phase elements CPE1 and CPE2, defined as 1/Y(jω)ni (where Y is a constant, ni is related to the angle of rotation of a purely capacitive line on the complex plane plots, ω is the angular frequency, and j equals to (−1)1/2), represent the double-layer capacitances generated from the ohmic and faradic processes, respectively.
As displayed in Fig. 5, the fitting results match well with the Nyquist plots. In addition, the chi-squared (χ2, which is the function defined as the sum of the squares of the residuals) of our correlation results is minimized below 10−4. All of these findings indicate that the equivalent circuit is reasonable and the calculated results are reliable. The calculated results obtained with the ZSimpWin simulation program are listed in Table 1.
| Sample | Rs (Ω) | R1 (Ω) | CPE1 − Y (mF cm−2)n1 | n1 | R2 (Ω) | CPE2 − Y (mF cm−2)n2 | n2 |
|---|---|---|---|---|---|---|---|
| RGO/Co3O4 (Eopc) | 0.70 | 0.99 | 0.51 | 0.83 | 113 | 9.01 | 0.81 |
| RGO/Co3O4/AB/M (Eopc) | 0.70 | 0.85 | 0.56 | 0.82 | 110 | 9.09 | 0.82 |
| RGO/Co3O4/AB/P (Eopc) | 0.70 | 0.84 | 0.56 | 0.82 | 109 | 9.11 | 0.82 |
| RGO/Co3O4/AB (Eopc) | 0.70 | 0.75 | 1.46 | 0.78 | 96 | 9.43 | 0.85 |
| RGO/Co3O4 (−0.2 V) | 0.69 | 1.03 | 0.50 | 0.79 | 7.48 | 8.39 | 0.91 |
| RGO/Co3O4/AB-M (−0.2 V) | 0.68 | 0.90 | 0.58 | 0.79 | 6.91 | 8.14 | 0.91 |
| RGO/Co3O4/AB-P (−0.2 V) | 0.68 | 0.89 | 0.59 | 0.79 | 6.82 | 8.03 | 0.91 |
| RGO/Co3O4/AB (−0.2 V) | 0.68 | 0.85 | 1.86 | 0.73 | 5.38 | 7.42 | 0.91 |
From Table 1, it can be found that with the addition of AB, the R1 and R2 values of the Co3O4/RGO/AB, Co3O4/RGO/AB-M and Co3O4/RGO/AB-P GDEs are lower in comparison with value of the RGO/Co3O4 GDE at both Eocp and −0.2 V, among which the R1 and R2 values of the Co3O4/RGO/AB GDE are by far the lowest. This further evidences the effective introduction strategy for AB and the rational structure of Co3O4/RGO/AB with increased catalytic sites and diffusion channels for the reactant molecules. It is worth noting that CPE1 − Y of the Co3O4/RGO/AB GDE is much higher than that of other GDEs, which can be ascribed to the much greater specific surface area. As for CPE2 − Y at Eocp, no ORR takes place, and the double-layer capacitance is caused by the adsorbed ions at the surface of GDE, leading to almost the same for CPE2 − Y. When the potential is −0.2 V, however, a larger number of ions adsorbed by Co3O4/RGO/AB are involved in ORR; therefore, the CPE2 − Y value of the Co3O4/RGO/AB GDE is the smallest of the GDEs. We also noticed that there are marginal differences in the fitting parameters between the Co3O4/RGO/AB-P and Co3O4/RGO/AB-M GDEs, implying the limitations of the ways of AB introduction for these two hybrids.
4. Conclusions
In summary, a Co3O4/RGO/AB hybrid has been successfully prepared through the introduction of AB concurrent with the synthesis of Co3O4/RGO via a simple one-step solution-phase route, which can effectively relieve the stacking of RGO sheets. Benefiting from the rational introduction strategy of AB and the corresponding hybrid nanostructure, the Co3O4/RGO/AB hybrid displays higher ORR catalytic activity than those of Co3O4/RGO, Co3O4/RGO/AB-P, Co3O4/RGO/AB-M and commercial 10 wt% Pt/C, which may be due to the large specific surface area, well-anchored Co3O4 nanoparticles on the RGO sheets, and low ohmic and kinetic impedances for ORR. We believe this work will be conducive for the extensive commercial applications of fuel cells.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (no. 21171174), Provincial Natural Science Foundation of Hunan (no. 09JJ3024), Provincial Environmental Science and Technology Foundation of Hunan, the fund of innovatively experimental project for undergraduate of Central South University (no. LC13077), the opening subject of State Key Laboratory of Powder Metallurgy, and the Open-end Fund for the Valuable and Precision Instruments of Central South University. We thank the Analysis and Testing Center of CSU for the HAADF and STEM-EDX characterization.Notes and references
- B. Wang, J. Power Sources, 2005, 152, 1–15 CrossRef CAS PubMed.
- R. Cao, J. S. Lee, M. Liu and J. Cho, Adv. Energy Mater., 2012, 2, 816–829 CrossRef CAS.
- Z. Chen, D. Higgins, A. Yu, L. Zhang and J. Zhang, Energy Environ. Sci., 2011, 4, 3167–3192 CAS.
- R. Bashyam and P. Zelenay, Nature, 2006, 443, 63–66 CrossRef CAS PubMed.
- G. Wu, K. L. More, C. M. Johnston and P. Zelenay, Science, 2011, 332, 443–447 CrossRef CAS PubMed.
- S. Yang, X. Feng, X. Wang and K. Mullen, Angew. Chem., 2011, 50, 5339–5343 CrossRef CAS PubMed.
- Y. Zhang, K. Fugane, T. Mori, L. Niu and J. Ye, J. Mater. Chem., 2012, 22, 6575–6580 RSC.
- R. Wang, J. Jia, H. Wang, Q. Wang, S. Ji and Z. Tian, J. Solid State Electrochem., 2012, 17, 1021–1028 CrossRef PubMed.
- Y. Ye, L. Kuai and B. Geng, J. Mater. Chem., 2012, 22, 19132–19138 RSC.
- Y. Liu, J. Ma, J. Lai and Y. Liu, J. Alloys Compd., 2009, 488, 204–207 CrossRef CAS PubMed.
- X. Yang, S. Li, Y. Liu, X. Wei and Y. Liu, J. Power Sources, 2011, 196, 4992–4995 CrossRef CAS PubMed.
- K. Lee, L. Zhang, H. Lui, R. Hui, Z. Shi and J. Zhang, Electrochim. Acta, 2009, 54, 4704–4711 CrossRef CAS PubMed.
- G. Wu, K. Artyushkova, M. Ferrandon, A. J. Kropf, D. Myers and P. Zelenay, ECS Trans., 2009, 25, 1299–1311 CAS.
- Y. Zhu, S. Murali, W. Cai, X. Li, J. W. Suk, J. R. Potts and R. S. Ruoff, Adv. Mater., 2010, 22, 3906–3924 CrossRef CAS PubMed.
- K. Kim, J. Y. Choi, T. Kim, S. H. Cho and H. J. Chung, Nature, 2011, 479, 338–344 CrossRef CAS PubMed.
- L. P. Biro, P. Nemes-Incze and P. Lambin, Nanoscale, 2012, 4, 1824–1839 RSC.
- B. Luo, B. Wang, M. Liang, J. Ning, X. Li and L. Zhi, Adv. Mater., 2012, 24, 1405–1409 CrossRef CAS PubMed.
- H. W. Ha, I. Y. Kim, S. J. Hwang and R. S. Ruoff, Electrochem. Solid-State Lett., 2011, 14, B70–B73 CrossRef CAS PubMed.
- T. N. Lambert, D. J. Davis, W. Lu, S. J. Limmer, P. G. Kotula, A. Thuli, M. Hungate, G. Ruan, Z. Jin and J. M. Tour, Chem. Commun., 2012, 48, 7931–7933 RSC.
- Y. Liang, H. Wang, J. Zhou, Y. Li, J. Wang, T. Regier and H. Dai, J. Am. Chem. Soc., 2012, 134, 3517–3523 CrossRef CAS PubMed.
- Z. S. Wu, S. Yang, Y. Sun, K. Parvez, X. Feng and K. Mullen, J. Am. Chem. Soc., 2012, 134, 9082–9085 CrossRef CAS PubMed.
- Y. Liang, Y. Li, H. Wang, J. Zhou, J. Wang, T. Regier and H. Dai, Nat. Mater., 2011, 10, 780–786 CrossRef CAS PubMed.
- Y. Si and E. T. Samulski, Chem. Mater., 2008, 20, 6792–6797 CrossRef CAS.
- C. N. Rao, A. K. Sood, K. S. Subrahmanyam and A. Govindaraj, Angew. Chem., 2009, 48, 7752–7777 CrossRef CAS PubMed.
- Y. Li, Y. Li, E. Zhu, T. McLouth, C. Y. Chiu, X. Huang and Y. Huang, J. Am. Chem. Soc., 2012, 134, 12326–12329 CrossRef CAS PubMed.
- H. Wang, Y. Liang, Y. Li and H. Dai, Angew. Chem., 2011, 50, 10969–10972 CrossRef CAS PubMed.
- S. Yang, K. Chang, Y. Huang, Y. Lee, H. Tien, S. Li, Y. Lee, C. Liu, C. M. Ma and C. Hu, Electrochem. Commun., 2012, 14, 39–42 CrossRef CAS PubMed.
- S. Yang, K. Chang, Y. Lee, C. M. Ma and C. Hu, Electrochem. Commun., 2010, 12, 1206–1209 CrossRef CAS PubMed.
- K. Chang, Y. Lee, C. Hu, C. Chang, C. Liu and Y. Yang, Chem. Commun., 2010, 46, 7957–7959 RSC.
- Y. Si and E. T. Samulski, Nano Lett., 2008, 8, 1679–1682 CrossRef CAS PubMed.
- D. Li, M. B. Muller, S. Gilje, R. B. Kaner and G. G. Wallace, Nat. Nanotechnol., 2008, 3, 101–105 CrossRef CAS PubMed.
- Y. Xu, K. Sheng, C. Li and G. Shi, J. Mater. Chem., 2011, 21, 7376–7380 RSC.
- G. Eda, J. Ball, C. Mattevi, M. Acik, L. Artiglia, G. Granozzi, Y. Chabal, T. D. Anthopoulos and M. Chhowalla, J. Mater. Chem., 2011, 21, 11217–11223 RSC.
- M. Bursell, M. Pirjamali and Y. Kiros, Electrochim. Acta, 2002, 47, 1651–1660 CrossRef CAS.
- J. Liu, J. Jiang, D. Qian, G. Tan, S. Peng, H. Yuan, D. Luo, Q. Wang and Y. Liu, RSC Adv., 2013, 3, 15457–15466 RSC.
- J. Yan, Z. Fan, T. Wei, W. Qian, M. Zhang and F. Wei, Carbon, 2010, 48, 3825–3833 CrossRef CAS PubMed.
- R. Kou, Y. Shao, D. Mei, Z. Nie, D. Wang, C. Wang, V. V. Viswanathan, S. Park, I. A. Aksay, Y. Lin, Y. Wang and J. Liu, J. Am. Chem. Soc., 2011, 133, 2541–2547 CrossRef CAS PubMed.
- R. Kou, Y. Shao, D. Wang, M. H. Engelhard, J. H. Kwak, J. Wang, V. V. Viswanathan, C. Wang, Y. Lin, Y. Wang, I. A. Aksay and J. Liu, Electrochem. Commun., 2009, 11, 954–957 CrossRef CAS PubMed.
- H. Huang, W. Zhang, M. Li, Y. Gan, J. Chen and Y. Kuang, J. Colloid Interface Sci., 2005, 284, 593–599 CrossRef CAS PubMed.
- C. C. Hu, S. C. Liao, K. H. Chang, Y. L. Yang and K. M. Lin, J. Power Sources, 2010, 195, 7259–7263 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |