Flexible TiO2/cellulose acetate hybrid film as a recyclable photocatalyst†
Xiujuan Jina,
Jing Xua,
Xianfu Wanga,
Zhong Xiea,
Zhe Liuab,
Bo Liangab,
Di Chen*a and
Guozhen Shen*b
aWuhan National Laboratory for Optoelectronics and School of Optical and Electronic Information, Huazhong University of Science and Technology, Wuhan 430074, China. E-mail: dichen@mail.hust.edu.cn; Fax: +86 27 87792225
bState Key Laboratory for Superlattices and Microstructures, Institute of Semiconductors, Chinese Academy of Sciences, Beijing 100083, China. E-mail: gzshen@semi.ac.cn
First published on 8th January 2014
Abstract
In this work, a flexible mesoporous TiO2 microspheres/cellulose acetate (TCA) hybrid film of tunable size and transparency was designed as a high performance recyclable photocatalyst. It was obtained by a simple method of dispersing mesoporous TiO2 microspheres onto the surface of a free-standing cellulose acetate (CA) film at room temperature. The photoelectrochemical properties of the mesoporous TiO2 microspheres were first studied by configuring them as a simple self-powered photoelectrochemical cell (PEC). Under UV light irradiation, the TCA hybrid film displays excellent flexibility and favorable recyclable photocatalytic activity for the decomposition of methylene blue (MB) solution. It was found that the pH value of the solution has a more significant effect than temperature on the photoactivity of the sample. Our results indicate that the hybrid film can be easily applied in the field of wastewater treatment without leaving any photocatalyst in the reaction system. It is feasible to develop this simple and environmentally friendly method to synthesize other catalyst systems such as WO3 film for potential industrial applications.
1. Introduction
Over the past few decades, environmental problems have attracted worldwide attention because of the increasingly worsening ecological environment crisis.1 Photocatalytic technology, as an efficient and green approach, has been widely researched for applications in wastewater purification, gas degradation and microdetection.2–4 Traditional powder photocatalysts usually need to be dispersed into wastewater, where they form suspensions to improve the reaction contact area. However, from a practical point of view, the additional separation step to remove the catalysts from the wastewater is inevitable. The use of catalysts as a slurry, after reaction, has created the problems of leaching and how to separate them from the system, which usually requires a long settlement time or centrifugation, and any residual toxic photocatalyst such as sulfide might cause secondary pollution.5,6 Hence, many researchers have suggested that this problem can be circumvented by supporting the catalyst on a suitable substrate.7–10 Until now, different methods, such as sol–gel, sputtering, electrospinning, plasma, or chemical vapor deposition have been used to deposit catalysts onto various substrates.11–14 Nevertheless, the intricate operations involved in depositing catalysts on a substrate, the expensive equipment needed, high-energy consumption, and environmental issues pose significant obstacles to their large-scale production and commercialization. On the other hand, direct mixing of the catalyst with a polymer significantly reduces the photocatalytic activity due to the decrease in available reaction area. Therefore, developing a convenient pathway for the synthesis of recyclable photocatalysts remains a great challenge.Cellulose acetate (CA) is biodegradable, nontoxic and biocompatible and can be prepared by esterification of cellulose, which is abundant in agricultural waste such as straw and biomass residues.15–17 These derivatized cellulose polymers have attracted considerable research interest as promising bio-based material candidates to replace the current widespread dependence on petroleum based materials, and to reduce the growing levels of environmental pollution resulting from the increasing amounts of non-biodegradable waste.18,19 Moreover, cellulose acetate has a certain mechanical strength that facilitates its processing into films, membranes, and fibers from either melts or solutions. Hence, with the help of the ripening technology available on the market, we can obtain various products at will.
TiO2 as a typical nontoxic,20 low cost, and efficient photocatalyst21 has also been widely used in many other fields, such as electrochemistry,22 high-performance hydrogen sensors,23,24 detoxification processes,25 dye-sensitized solar cells and so on.26–28 In recent years, mesoporous TiO2 nanoparticles have played and will continue to play an important role in photocatalytic applications due to their high specific surface area, which is beneficial for promoting the diffusion of reactants and products, as well as for enhancing photocatalytic activity by facilitating access to reactive sites on the surface of the photocatalyst.29,30 If one can connect CA to TiO2 powder, some devices with interesting properties may be obtained that would suit different applications, such as solving pollution problems.
In the present work, we report a simple method for the fabrication of TiO2/cellulose acetate (TCA) hybrid film, which serves as recyclable photocatalyst. As expected, the TCA hybrid film can be easily reclaimed without a decrease in the activity for the degradation of MB solution. The TCA hybrid film with its flexible, transparent and environment-friendly properties, can be used as a self-cleaning material, or in other applications. Moreover, as the properties of the composite film are mainly determined by the catalyst surface, we can change the composite materials to obtain various films at will. For example, by replacing TiO2 with WO3, we can use the film to degrade pollutants during wastewater treatment under visible-light irradiation. Additionally, cellulose acetate can be substituted with other polymers for some specialist applications.
2. Experimental section
2.1. Materials
All chemical reagents were of analytical grade and used without further purification, including tetrabutyl titanate (TBT), polyethylene glycol (PEG, MW 2000), glacial acetic acid (HAC), ethanol (C2H5OH), muriatic acid (HCl), urea (CO(NH2)2), cellulose acetate (CA) and methylene blue trihydrate (MB).2.2. Synthesis and characterization of TiO2 mesoporous microspheres
TBT (1 mL), HCl (0.5 mL), and HAC (10 mL) were dissolved in ethanol (15 mL) to form a clarified solution. Then PEG2000 (0.2 g) and urea (0.5 g) were added. The mixture was vigorously stirred for 30 min with a magnetic stirrer at room temperature until a transparent solution was obtained. The mixture was transported into a Teflon-lined stainless steel autoclave (50 mL capacity), kept at 180 °C for 12 h, and then cooled to ambient temperature naturally. Finally, the resulting white product was collected, washed with distilled water and ethanol several times, then dried in air at 70 °C for 4 h. The collected powder was further heated in a muffle furnace at 550 °C for 4 h to obtain pure TiO2 mesoporous microspheres.X-ray diffraction patterns (XRD) were obtained from a X-ray diffractometer (X'Pert PRO, PANalytical B.V., the Netherlands) with radiation of a Cu target (Ka, λ = 0.15406 nm). The field emission scanning electron microscopy (FESEM) images (JEOL JSM-6700F, 5 kV) revealed the morphologies of the samples. Nitrogen adsorption–desorption was measured using a ASAP 2020 accelerated surface area and porosimetry system at −196 °C. The Brunauer–Emmett–Teller (BET) method was used to calculate the specific surface area. The pore size distribution of the materials was derived from the adsorption branches of the isotherms based on the Barrett–Joyner–Halenda (BJH) model. The UV-VIS absorption spectra were obtained using a Shimadzu UV-2550 spectrophotometer. The pH value of the solution was measured by a pH meter (pHS-3C, ECS002137). FT-IR spectra were recorded using a VERTEX-70 FT-IR spectrometer in the range 4000–400 cm−1.
2.3. Photoelectrochemical properties measurements
In this work, the photoelectrochemical properties of the TiO2 spheres were investigated by employing a simple and self-powered photoelectrochemical cell (PEC) without an external bias. For the photoelectrode, a film of TiO2 spheres prepared using a typical Solaronix T-paste was coated on a clean fluorine-doped tin oxide (FTO) glass by the well-known doctor-blade technique developed for DSSCs. Then the film was sintered at 450 °C in air for half an hour.31,32 The dried photoelectrode was sealed using a 25 μm thick Surlyn gasket melted by heating with the Pt counter-electrode. The latter was prepared by dropping 5 mM H2PtCl6 ethanol solution on the FTO glass, followed by heating at 400 °C for 30 min under air. The internal space of the cell was filled with an electrolyte consisting of DMPII (1.0 M), LiI (0.1 M), I2 (0.12 M), and 4-TBP (0.5 M) in methoxypropionitrile by a vacuum backfilling system. The illuminated area was 0.49 cm2. All measurements were performed at room temperature. IPCE was measured by an IPCE testing system (Newport), and the short-circuit current as a function of time at 365 nm was measured using a Keithley 2400 multimeter. An ultraviolet hand lamp with a power density of 1.25 mW cm−2 (λ = 365/254 nm switchable) was used.2.4. Fabrication of the TiO2/cellulose acetate hybrid film
Typically, cellulose acetate powders (3 g) were poured into HAC (10 mL) in a beaker, and then sealed with parafilm. Transparent CA gelatum was formed after aging for one day. At the same time, the as-prepared TiO2 powder (0.01 g) was uniformly dispersed into HAC (1 mL) by ultrasonic treatment for 30 min. Then, the CA gelatum was coated on glass (6 cm × 6 cm) by the doctor-blade technique. It is noted that the size of the film can be easily tuned for different purposes. Following this, the above TiO2 solution was pasted into CA by the same method. After several hours, the CA gelatum solidified with TiO2 microspheres had partly cohered onto the surface and had formed a composite film, which was denoted as the TCA hybrid film. The reference CA film was fabricated under the same conditions without the addition of TiO2 powder. The thickness of the CA film was easily controlled by adjusting the layers of sellotape. All operations were carried out in a ventilation hood at room temperature.2.5. Photocatalytic activity measurements
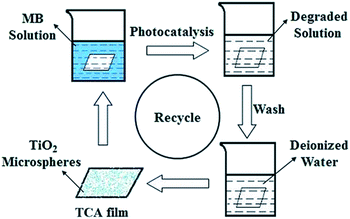
Degradation of MB solution was carried out to investigate the photocatalytic properties of the as-prepared TCA hybrid film. The film, of size of 4 × 6 cm, was soaked in identical beakers containing MB solution (50 mL) with an initial concentration of 4.0 mg L−1, and stored in the dark without stirring for 0.5 h to reach an adsorption–desorption equilibrium. The reaction system was then illuminated by a 500 W Hg lamp with a 7.5 A current at room temperature, with the TiO2 facing the UV light in order to receive as much UV radiation as possible. In the contrast experiments, an ultraviolet hand lamp with a power density of 1.25 mW cm−2 (λ = 365/254 nm switchable) was used. Intermediate solutions were collected every hour to test the remaining concentration of MB using a Shimadzu UV-2550 spectrophotometer. Beakers were placed in larger beakers with an ice-water mixture to realize the reference tests at 0 °C. After photocatalytic activity measurements, the TCA hybrid film was immersed in deionized water for 2 h to remove residual ions and molecules. Then, the film sample was again put into the MB solution to study its recyclability. From the degradation rates obtained the stability of the catalyst was evaluated. As shown in Scheme 1, the process was repeated three times to ensure recycling stability.3. Results and discussion
3.1. Characterization of the mesoporous TiO2 microspheres
A typical SEM image of the as-synthesized TiO2 sample is shown in Fig. 1a, which revealed that the sample contains uniform TiO2 microspheres with an average diameter of about 3 μm. The high magnification SEM image (Fig. 1b) revealed that the surface of the TiO2 microsphere is rough with many flower-like wrinkles. Fig 1c gives a clearer view of the surface, where many fine particles with diameters of about 20 nm can be easily seen. X-ray diffraction (XRD) was used to identify the crystal structure and phase compositions of the as-prepared TiO2 sample. As shown in Fig. 1d, all peaks in the XRD pattern could be indexed to the anatase phase structure (JCPDS no. 84-1286). No peaks from other phases were detected. The strong and sharp diffraction peaks indicated the good crystallinity of the as-synthesized product.The mesoporous nature of the TiO2 spheres is further confirmed by Brunauer–Emmett–Teller (BET) measurements and the N2 adsorption–desorption isotherms are shown in Fig. 2. The sample displayed a typical type-IV isotherm curve with an obvious hysteresis loop at high relative pressure (P/P0 = 0.78–0.95), indicating the presence of mesoporous microspheres. The plot of the pore size distribution is determined by Barrett–Joyner–Halenda (BJH) analysis of the desorption branch of the isotherm (inset of Fig. 2). The BET surface area of the TiO2 spheres is 37.9 m2 g−1 and the pore size distribution centre is at 11.2 nm. The large surface area and porosity will contribute to the high adsorption of dye.
 | ||
| Fig. 2 N2 adsorption–desorption isotherms. The inset shows the corresponding pore size distribution plot. | ||
3.2. Photoelectrochemical properties of the mesoporous TiO2 microspheres
A two-electrode photoelectrochemical cell (PEC) based on the as-synthesized mesoporous TiO2 microspheres with FTO as a substrate was employed to investigate the photoelectrochemical properties without a bias voltage. As shown, photons with energy higher than the TiO2 bandgap energy are absorbed by the TiO2 microspheres accompanied by the generation of electron–hole pairs. The holes accept electrons from the redox electrolyte species (I−), and the electrons are transported through the mesoporous TiO2 to the anode contact. At the same time, oxidized redox ions (I3−/I−) diffuse to the cathode, where they are reduced by electrons that have passed through the external circuit when a photocurrent flows. The incident-photon-to-current-conversion efficiency (IPCE) is recognized as a good parameter to characterize the photoconversion efficiency.33,34 Fig. 3a displays the photocurrent action spectrum in which the IPCE is plotted as a function of the excitation wavelength. The PEC cell obtained the highest efficiency, 9.73%, at a wavelength of around 345 nm, which indicates that the material we have obtained may be an efficient catalyst under UV light irradiation.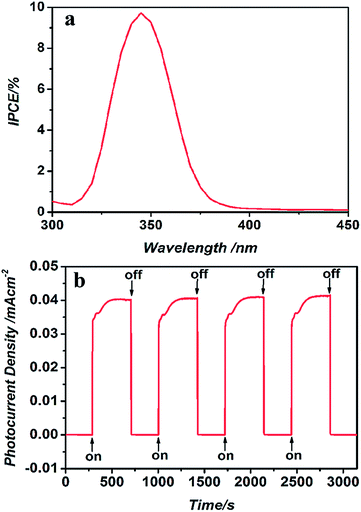 | ||
| Fig. 3 (a) IPCE spectrum of the PEC made up of mesoporous TiO2 microspheres, (b) the photocurrent–time characteristics during light “on–off” cycles of the PEC at wavelength 365 nm. | ||
To further investigate the photoactivity and stability of the as-prepared TiO2, the short-circuit current as a function of time was measured at 365 nm. As shown in Fig. 3b, the photocurrent can be reproducibly switched from the “on” state to the “off” state by periodical turning of the UV light source on and off with a power density of 1.25 mW cm−2 at wavelength 365 nm. Upon illumination by UV light, the photocurrent was promptly increased to 0.032 mA cm−2, and then gradually increased with extending irradiation time during the light “on” process, indicative of a photoreaction occurring in the cell. After irradiation for about 180 s, the photocurrent reached a steady state (0.416 mA cm−2). The current was drastically decreased to its initial level of 1.614 × 10−6 mA cm−2 when the light was turned off. The remarkable IPCE and photocurrent observed reflected the efficient generation/separation of photo-induced electrons and holes in the TiO2 microspheres as well as steady recyclability, which suggests that these microspheres are a promising photocatalytic material.
3.3. Flexible TiO2/cellulose acetate hybrid film (TCA hybrid film)
To obtain efficient and environment-friendly photocatalytic materials, free-standing and flexible TCA hybrid films were prepared by the simple doctor-blade process. One side of the TiO2 microspheres was exposed to air, and the other side adhered to the surface of the CA film substrate, making a firm mechanical joint between the CA film and the TiO2 particles. For comparison, pure CA film was also fabricated in a similar way but without the use of TiO2 microspheres. The corresponding optical images of the CA film and the TCA film containing about 0.01 g of prepared TiO2 are shown in Fig. 4, which intuitively show the high transparency (top) and flexibility (bottom). The size of the films could be easily controlled by adjusting the sample die, and the transparency can also be easily controlled by optimizing the thickness of the CA film and the amount of dispersed TiO2 spheres. These features make it a promising candidate for some potentially flexible and transparent devices.The structural stability of the CA was maintained during the film preparation process. It was confirmed by comparing the infrared spectra of the as-received CA powder to the obtained TCA film. Fig. 5 shows the FT-IR spectra of CA powder, CA film and the TCA hybrid film, and the inset is the chemical structure of the as-received CA powder. All spectra show that a number of absorption features are below 2000 cm−1. These major absorption features appeared at 1732 cm−1 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1451 cm−1 (O
O), 1451 cm−1 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OR), 1369 cm−1 (–CH2), 1217 cm−1 (C–O), 1032 cm−1 (C–O–C) and 900 cm−1 (–CH).35–37 Features above 2000 cm−1 are both intense and composition sensitive. They appeared at 2922 cm−1 (–CH2) and 3700–3200 cm−1 (–OH) characteristic bands of CA, especially in the presence of both –OH and the characteristic stretching bands at 3700–3200 cm−1. All three samples exhibited similar characteristic features without introducing any new peaks. The results demonstrated that only physical blending and no chemical reaction occurred between the TiO2 and CA film.
C–OR), 1369 cm−1 (–CH2), 1217 cm−1 (C–O), 1032 cm−1 (C–O–C) and 900 cm−1 (–CH).35–37 Features above 2000 cm−1 are both intense and composition sensitive. They appeared at 2922 cm−1 (–CH2) and 3700–3200 cm−1 (–OH) characteristic bands of CA, especially in the presence of both –OH and the characteristic stretching bands at 3700–3200 cm−1. All three samples exhibited similar characteristic features without introducing any new peaks. The results demonstrated that only physical blending and no chemical reaction occurred between the TiO2 and CA film.
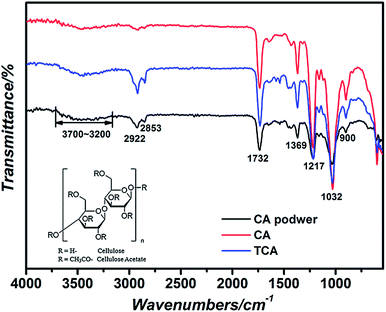 | ||
| Fig. 5 FT-IR spectra of CA powder, CA film and TCA hybrid film. Inset: chemical structure of the as-received CA powder. | ||
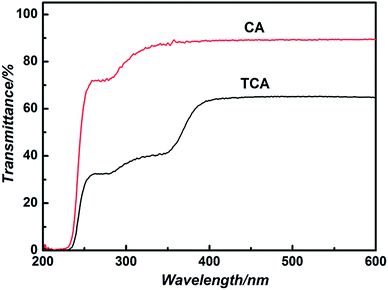
The UV-vis transmission and absorption spectra of the CA film and the TCA film were also measured with a Shimadzu UV-2550 spectrophotometer. The transparencies of the as-prepared films were investigated from the transmission spectra (Fig. 6). It was clear that the CA film and the TCA hybrid film perform high transmittance up to nearly 90% and 60%, respectively, which are in good agreement with the optical photographs in Fig. 4, indicating that both the CA film and the TCA hybrid film have excellent transparencies. Fig. S1† shows that the flexible and transparent CA film can only absorb light in the region below 300 nm. In contrast, the TCA hybrid film exhibits broader absorption in the range from 200 to 400 nm. This obvious red-shift indicates that the addition of TiO2 effectively utilizes more light.
3.4. Photocatalytic activity of the TCA hybrid film
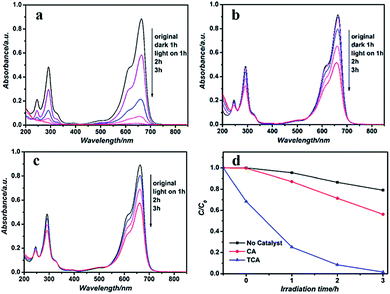
When investigating photocatalytic properties it is quite conventional to use MB as the pollutant .38–40 The Houas group investigated the detailed TiO2/UV photocatalytic degradation pathway of MB. In addition to the prompt removal of color, the TiO2/UV-based photocatalyst was simultaneously able to oxidize the dye, with an almost complete mineralization of carbon and nitrogen and sulfur heteroatoms into CO2, NH4+, NO3− and SO42−, respectively. In our study, we chose the degradation of MB solution as a model reaction to evaluate the photocatalytic activity of the TCA hybrid film. As shown in Scheme 1, a uniform TCA hybrid film (4 cm × 6 cm) containing about 0.01 g of the prepared TiO2 powder was immersed, firstly, into the blue dye solution (4 mg L−1) to capture MB molecules under dark reaction. When the system reached an adsorption–desorption equilibrium, UV light was turned on. After 3 h of irradiation, the color of the degraded solution had changed from blue to white and the TCA hybrid film also became white in color. In Xu's work,41 the photocatalytic activity of ZnO powder on the degradation of MB under UV light irradiation was investigated. In this experiment, the dosage of ZnO powder was 0.1 g corresponding to 0.01 g of TiO2 in our work, and the other experimental conditions were kept the same. 91.08% of MB was converted after 70 min of irradiation in the ZnO nanorod photoreaction system. It is well known that, as well as TiO2, ZnO is a promising UV-light photocatalyst. Hence, almost all MB was degraded in 3 h by the TCA film with only 0.01 g TiO2, indicating the excellent photocatalytic activity of the hybrid film. Certainly, we can increase the amount of TiO2 in the TCA film to improve the photocatalytic properties at the cost of transparency. Furthermore, the TCA hybrid film could be easily removed from the dye solution. Afterwards, for cleaning the film was soaked in a distilled water solution to remove possible residual ions and molecules, and could be used repeatedly.Generally, the photocatalysis process consisted of two parts: absorption and degradation. First, the dye is absorbed on the surface of the photocatalyst. Subsequently, the reaction of the photogenerated holes with water molecules and hydroxyl ions adsorbed on the surface of TiO2 yielded the formation of hydroxyl radicals (˙OH), capable of mineralizing organic compounds to carbon dioxide and water.42–44 The degradation efficiency of the as-prepared sample was defined as C/C0, where C and C0 stand for the residual and initial concentration of MB, respectively. Fig. 7a shows the UV-vis spectra changes of MB solutions over the TCA hybrid film during the degradation processes. Clearly, with an increase of irradiation time, not only the main absorption peaks in visible region but also the peaks in the UV region decrease dramatically, indicating that the dye molecules have been degraded instead of being simply decolorized. After 3 h, the MB solution was colorless and the MB absorption peaks had almost disappeared, suggesting the excellent photocatalytic activity of the TCA hybrid film. For comparison, the photocatalytic activities of pure CA film without the TiO2 microspheres was further investigated for MB solution degradation under UV light irradiation and the results are shown in Fig. 7b. It was obvious that the adsorption and photocatalytic abilities of the film decreased under the same experimental conditions. A blank experiment, photolysis of MB in the absence of any photocatalyst but under UV light irradiation was also performed. As shown in Fig. 7c, photolysis was very low, and nearly 80% of MB was retained after 3 h irradiation. Fig. 6d shows the concentration changes of MB under various photodegradation reaction systems determined by its characteristic absorption peak (at 664 nm), to give a visual comparison of the various degradation rates. Firstly, by comparing the photolysis of the MB solution with the reaction in the presence of the TCA hybrid film, it is obviously a photo-driven degradation process under the light irradiation. Then, the TCA hybrid film shows stronger adsorption and photocatalytic degradation abilities for MB in comparison to CA film under the same conditions. During the dark reaction, around 40% MB molecules were adsorbed onto the TCA hybrid film due to the presence of TiO2, while only 0.01% MB dyes were adsorbed onto the pure CA film. Under irradiation by UV light for 3 h, the TCA hybrid film exhibited superior photocatalytic activity. For a better comparison of the photocatalytic efficiency with different reaction systems, the kinetic linear simulation curves of MB photocatalytic degradation are shown in Fig. S2.† Obviously, the TCA hybrid film with the largest rate constant results in the highest catalytic activity. To further confirm that the decrease of MB concentration is mainly due to photocatalytic effects, instead of photolysis, weaker UV light sources with a power density of 1.25 mW cm−2 at wavelengths 365 nm and 254 nm were used. Fig. S3† shows the changes of MB solution concentration under different light sources in the presence of TCA hybrid film. Under irradiation by an UV-365 nm LED lamp, more than 80% of MB was converted in 3 h, while only 40% of MB solution was degraded under irradiation by an UV-254 nm LED lamp. Since the energy of 365 nm light is a better match with the TiO2 energy band obtained, we concluded that the degradation of MB in our reaction is a photo-driven catalytic reaction process in the presence of a catalyst.
The degradation of non-colored phenol was also run to further ensure the photocatalytic activity of our hybrid photocatalysts. Considering that the excellent property of the hybrid TCA film is mainly affected by the photocatalytic nature of the loaded TiO2 microspheres, the as-synthesized TiO2 spheres were thus selected as the catalysts. In the experiment, 0.1 g TiO2 was added to a reactor containing 100 mL phenol solution with an initial concentration of 50 mg L−1. The reaction system was illuminated by a Hg lamp. From Fig. 8a, we found that almost no degradation occurred without a catalyst. Fig. 8b and c show the UV-vis spectral changes of the phenol solution during the degradation processes with 0.1 g P25 and the as-grown TiO2 spheres as the catalysts, respectively. The results clearly revealed that TiO2 spheres have better performance than the commercial P25 catalyst. Fig. 8d shows the concentration changes of phenol in various photodegradation reaction systems determined from its characteristic absorption peak (at 270 nm) to give a visual comparison of the corresponding degradation rates. Comparing with the photolysis of phenol solution, the reaction in the presence of the catalyst is an obvious photo-driven degradation process under light irradiation. Besides, the as-obtained TiO2 spheres possess higher photocatalytic activity than commercial P25, which is consistent with the MB degradation results.
Interestingly, the CA film shows a certain photocatalytic ability (Fig. 7b) in the degradation of MB compared to the photolysis process, which has hardly been reported. Considering that the CA film in aqueous solution could release HAC with time, the pH value changes with reaction time were examined in photodegradation reaction systems. Fig. 9a shows the pH value changes of MB and CA film in aqueous solution with time in the dark. The pH values of MB solution remained rather stable at about 5.7 in three hours, but those of the CA solution exhibited an obvious decrease ranging from 5.45 to 4.3, indicating hydrolyzation of the CA products. Afterwards, experiments of MB photodegradation at various pH values without any catalyst were conducted to research the influence of acidity. In these cases, the pH of the system was adjusted by adding an appropriate HCl solution. The effect of pH value on the MB degradation is shown in Fig. 9b. The degradation rate of MB at pH = 5.5 is higher than at pH = 4.0, which suggests that a lower pH value may have a certain inhibitory action on MB degradation. Hence, pH value does not stimulate the CA film photoreaction system. On the other hand, during the irradiation process, the temperature of the photodegradation reaction systems rose, which may be an interference factor in the degradation of MB solution. A reference experiment of MB irradiated by UV light at zero degrees with the help of an ice and water mixture was conducted. It can be seen from Fig. 9b that the degradation of MB without catalyst at various temperatures shows only slight differences.
 | ||
| Fig. 9 (a) pH value changes of MB solution and CA film with reaction time in the dark, and (b) MB decomposition upon UV irradiation at different pH and temperature. | ||
Hence, the influence of temperature on degradation could also be removed. Besides, the long term stability of the CA film in water under irradiation by UV light was also investigated. UV-vis spectra of aqueous solutions with CA film at different irradiation times are shown in Fig. S4,† and the spectra are similar to HAC. The absorption peak intensity was enhanced at 190–240 nm with the increase of irradiation time, especially in the first 1 h, and then increased slowly, which corresponded to the changes in pH value, shown in Fig. 9a. After 4 h, the peak intensity changed little, indicating that the solution composition tended towards stability. In the present case, it can be presumed that the CA film itself has a certain photocatalytic degradation effect on MB, which is signally enhanced by the addition of TiO2 on its surface. Moreover, the good stability of CA film in water is beneficial to the recyclability of the TCA hybrid film.
To practically demonstrate the recyclability of the TCA hybrid film which served as the photocatalyst in the degradation of MB, a TCA hybrid film containing only about 0.01 g of prepared TiO2 powder was used. As shown in Scheme 1, the film was first immersed into the blue solution to capture MB molecules (4.0 mg L−1), which was followed by irradiation with UV light for 3 h to degrade the organic dye. Afterwards, the TCA hybrid film was removed from the system and soaked in deionized water for 2 h to wipe off possible residual ions and molecules. Then, the film was transferred into a new dye system for another cycle of degradation. Fig. 10 shows that, after cycling three times, the photocatalytic degradation rates of MB over TCA hybrid film under UV light irradiation hardly decreased, which was in good agreement with the photoactivity measurement shown in Fig. 3b. The irradiation-lifting-washing process can be repeated multiple times, while retaining high photocatalytic activity. This indicates that the TCA film with high photocatalytic activity can be easily recycled and so greatly promote its use in industrial applications to eliminate organic pollutants from wastewater.
 | ||
| Fig. 10 Photocatalytic activity of the sample TCA hybrid film on MB degradation with recycling (three times). | ||
4. Conclusions
In this study, by using a modified solvothermal process anatase TiO2 mesoporous microspheres, that served as high activity photocatalysts, were successfully synthesized. The photoelectrochemical properties of the sample were measured by employing a simple two-electrode PEC without a bias. The PEC displayed a steady and obvious photocurrent response under irradiation of 365 nm UV light. Moreover, a flexible and environmentally friendly TiO2/cellulose acetate hybrid film of tunable size and transparency was fabricated using a simple doctor-blade method, that achieved its main purpose which was to degrade organic pollutants without leaving any photocatalyst in the system to cause secondary pollution. After several rounds of recycling, the degradation rate of MB solution under UV light irradiation with TCA hybrid film remained at almost the same level as the first cycle time, suggesting that this convenient and green technique for the fabrication of TCA hybrid film could lead to an efficient recyclable photocatalyst. Interestingly, after eliminating the possible effects of pH value and temperature, we found that the CA film has certain photocatalytic activities for MB solution. Furthermore, this technique could also be applied to other polymer/nanoparticle systems and paves the way for other novel advanced recyclable photocatalytic materials.Acknowledgements
This work was supported by the National Natural Science Foundation (91123008, 61377033), the 973 Program of China (no.2011CB933300), the Program for New Century Excellent Talents of the University in China (grant no. NCET-11-0179). We also appreciate the Analytical and Testing Center of HUST and the Center of Micro-Fabrication and Characterization (CMFC) of WNLO for the use of their facilities.Notes and references
- T. Abe and M. Kaneko, Prog. Polym. Sci., 2003, 28, 1441 CrossRef CAS.
- D. Chen, S. X. Ouyang and J. H. Ye, Nanoscale Res. Lett., 2009, 4, 274 CrossRef CAS PubMed.
- G. Z. Shen, P. C. Chen and C. W. Zhou, J. Mater. Chem., 2009, 19, 828 RSC.
- D. Chen and J. H. Ye, Adv. Funct. Mater., 2008, 18, 1922 CrossRef CAS.
- P. Chen, L. Gu, X. D. Xue, M. J. Li and X. B. Cao, Chem. Commun., 2010, 46, 5906 RSC.
- B. Neppolian, H. C. Choi, S. Sakthivel, B. Arabindoo and V. Murugesan, J. Hazard. Mater., 2002, 89, 303 CrossRef CAS.
- N. J. Peill and M. R. Hoffmann, Environ. Sci. Technol., 1995, 29, 2974 CrossRef CAS PubMed.
- J. Sabate, M. A. Anderson, H. Kikkawa, M. Edwards and J. C. G. Hill, J. Catal., 1991, 127, 167 CrossRef CAS.
- A. Fernandz, G. Lasaletta, V. M. Jimenez, A. Justo, A. R. Gonzalez-elipe, J. M. Herrmann, H. Tahiri and Y. Ait-Ichon, J. Appl. Catal. B: Environ., 1995, 7, 49 CrossRef.
- K. Tennakone, C. T. K. Tilakaratne and I. R. M. Kotegoda, J. Photochem. Photobiol., A, 1995, 87, 177 CrossRef CAS.
- A. Bozzi, T. Yuranova and J. Kiwi, J. Photochem. Photobiol., A, 2005, 172, 27 CrossRef CAS PubMed.
- J. Verran, A. Packer, P. Kelly and K. A. Whitehead, Int. J. Food Microbiol., 2010, 141, S134 CrossRef CAS PubMed.
- N. M. Bedford and A. J. Steckl, ACS Appl. Mater. Interfaces, 2010, 2, 2445 Search PubMed.
- A. Bozzi, T. Yuranova, I. Guasaquillo, D. Laub and J. Kiwi, J. Photochem. Photobiol., A, 2005, 174, 156 CrossRef CAS PubMed.
- J. Puls, S. A. Wilson and D. Hölter, J. Polym. Environ., 2011, 19, 152 CrossRef CAS.
- N. Y. Abou-zeid, A. I. Waly, N. G. Kandile, A. A. Rushdy, M. A. El-Sheikh and H. M. Ibrahim, Carbohydr. Polym., 2011, 84, 223 CrossRef CAS PubMed.
- D. Wang, G. Sun and L. W. Yu, Carbohydr. Polym., 2011, 83, 1095 CrossRef CAS PubMed.
- B. E. Dale, J. Chem. Technol. Biotechnol., 2003, 78, 1093 CrossRef CAS.
- A. K. Mohanty, M. Mirsa and L. T. Drzal, J. Polym. Environ., 2002, 10, 19 CrossRef CAS.
- J. Zhang, Q. Xu, Z. C. Feng, M. J. Li and C. Li, Angew. Chem., 2008, 47, 1766 CrossRef CAS PubMed.
- Z. Q. He, X. Xu, S. Song, L. Xie, J. J. Tu, J. M. Chen and B. Yan, J. Phys. Chem. C, 2008, 112, 16431 CAS.
- M. Tian, G. S. Wu, B. Adams, J. L. Wen and A. C. Chen, J. Phys. Chem. C, 2008, 112, 825 CAS.
- O. K. Varghese, D. W. Gong, M. Paulose, K. G. Ong, E. C. Dickey and C. A. Grimes, Adv. Mater., 2003, 15, 624 CrossRef CAS.
- O. K. Varghese, D. W. Gong, M. Paulose, K. G. Ong and C. A. Grimes, Sens. Actuators, B, 2003, 93, 338 CrossRef CAS.
- Z. H. Zhang, Y. Yuan, G. Y. Shi, Y. J. Fang, L. H. Liang, H. C. Ding and L. T. Jin, Environ. Sci. Technol., 2007, 41, 6259 CrossRef CAS.
- L. E. Greene, M. Law, B. D. Yuhas and P. D. Yang, J. Phys. Chem. C, 2007, 111, 18451 CAS.
- D. Kim, A. Ghicov, S. P. Albu and P. Schmuki, J. Am. Chem. Soc., 2008, 130, 16454 CrossRef CAS PubMed.
- T.-S. Kang, A. P. Smith, B. E. Taylor and M. F. Durstock, Nano Lett., 2009, 9, 601 CrossRef CAS PubMed.
- A. A. Ismail and D. W. Bahnemannb, J. Mater. Chem., 2011, 21, 11686 RSC.
- N. Li, G. Liu, C. Zhen, F. Li, L. L. Zhang and H. M. Cheng, Adv. Funct. Mater., 2011, 21, 1717 CrossRef CAS.
- G. P. Smestad and M. Grätzel, J. Chem. Educ., 1998, 75, 752 CrossRef CAS.
- E. V. Vigil, L. M. Peter, F. Forcade, J. R. Jennings, B. González, H. X. Wang, L. Curbelo and H. Dunn, Sens. Actuators, A, 2011, 171, 87 CrossRef CAS PubMed.
- G. M. Wang, H. Y. Wang, Y. C. Ling, Y. C. Tang, X. Y. Yang, R. C. Fitzmorris, C. C. Wang, J. Z. Zhang and Y. Li, Nano Lett., 2011, 11, 3026 CrossRef CAS PubMed.
- Z. R. Wang, H. Wang, B. Liu, W. Z. Qiu, J. Zhang, S. H. Ran, H. T. Huang, J. Xu, H. W. Han, D. Chen and G. Z. Shen, ACS Nano, 2011, 5, 8412 CrossRef CAS PubMed.
- Y. E. Greish, M. A. Meetani, E. A. Al Matroushi and B. A. Shamsi, Carbohydr. Polym., 2010, 82, 569 CrossRef CAS PubMed.
- B. Ding, E. Kimura, T. Sato, S. Fujita and S. Shirator, Polymer, 2004, 45, 1895 CrossRef CAS PubMed.
- J. H. Ryu, H. J. Lee, Y. J. Kim, Y. S. Kang and H. S. Kim, Chem. – Eur. J., 2001, 7, 1525 CrossRef CAS.
- Y. J. Acosta-Silva, R. Nava, V. Hernández-Morales, S. A. Macías-Sánchez, M. L. Gómez-Herrera and B. Pawelec, Appl. Catal., B, 2011, 110, 108 CrossRef CAS PubMed.
- A. Houas, H. Lachheb, M. Ksibi, E. Elaloui, C. Guillard and J. Herrmann, Appl. Catal., B, 2001, 31, 145 CrossRef CAS.
- G. Lui, J. Y. Liao, A. S. Duan, Z. S. Zhang, M. Fowler and A. P. Yu, J. Mater. Chem. A, 2013, 1, 12255 CAS.
- J. Xu, Y. G. Zhu, H. T. Huang, Z. Xie, D. Chen and G. Z. Shen, CrystEngComm, 2011, 13, 2629 RSC.
- J. Li, S. L. Zhou, G. B. Hong and C. T. Chang, Chem. – Eur. J., 2013, 219, 486 CAS.
- J. L. Hu, H. S. Qian, Y. Hu, Z. Q. Li, G. X. Tong, T. K. Ying, P. J. Gong, S. Y. Hao, H. B. Zhang and L. C. Li, CrystEngComm, 2012, 14, 7118 RSC.
- S. Chaguetmi, F. Mammeri, S. Nowak, P. Decore, H. Lecoq, M. Gaceur, J. B. Naceur, S. Achour, R. Chtourou and S. Ammar, RSC Adv., 2013, 3, 2572 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra47710j |
| This journal is © The Royal Society of Chemistry 2014 |