Cyclododecanone as a recyclable protecting group for the synthesis of highly functionalized 3-amino-2-thiohydantoins from conventional starting materials†
Abstract
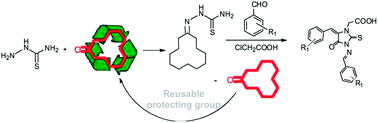
A simple and efficient approach has been developed for the synthesis of highly substituted 3-amino-2-thiohydantoins using cyclododecanone as a protecting group and thiosemicarbazide, chloroacetic acid and substituted benzaldehyde as reactants. This high yielding (79–96%) protocol is a milder alternative to the traditional method that uses unprotected thiosemicarbazide. It was identified that the preferred pathway is the deprotection of 3-amino-2-thiohydantoins followed by the attack of chloroacetic acid on a secondary nitrogen with subsequent formation of the Knoevenagel product and its reaction with imine leading to the final product.

 Please wait while we load your content...
Please wait while we load your content...